Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis
- PMID: 16009715
- PMCID: PMC2213006
- DOI: 10.1084/jem.20041685
Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis
Abstract
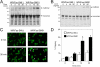
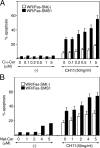
Engagement of the Fas receptor (CD95) initiates multiple signaling pathways that lead to apoptosis, such as the formation of death-inducing signaling complex (DISC), activation of caspase cascades, and the generation of the lipid messenger, ceramide. Sphingomyelin (SM) is a major component of lipid rafts, which are specialized structures that enhance the efficiency of membrane receptor signaling and are a main source of ceramide. However, the functions of SM in Fas-mediated apoptosis have yet to be clearly defined, as the responsible genes have not been identified. After cloning a gene responsible for SM synthesis, SMS1, we established SM synthase-defective WR19L cells transfected with the human Fas gene (WR/Fas-SM(-)), and cells that have been functionally restored by transfection with SMS1 (WR/Fas-SMS1). We show that expression of membrane SM enhances Fas-mediated apoptosis through increasing DISC formation, activation of caspases, efficient translocation of Fas into lipid rafts, and subsequent Fas clustering. Furthermore, WR/Fas-SMS1 cells, but not WR/Fas-SM(-) cells, showed a considerable increase in ceramide generation within lipid rafts upon Fas stimulation. These data suggest that a membrane SM is important for Fas clustering through aggregation of lipid rafts, leading to Fas-mediated apoptosis.
Figures






Similar articles
-
Impaired TCR signaling through dysfunction of lipid rafts in sphingomyelin synthase 1 (SMS1)-knockdown T cells.Int Immunol. 2008 Nov;20(11):1427-37. doi: 10.1093/intimm/dxn100. Epub 2008 Sep 26. Int Immunol. 2008. PMID: 18820264
-
Caspase-mediated inhibition of sphingomyelin synthesis is involved in FasL-triggered cell death.Cell Death Differ. 2010 Apr;17(4):642-54. doi: 10.1038/cdd.2009.130. Epub 2009 Sep 25. Cell Death Differ. 2010. PMID: 19779494
-
CD95 death-inducing signaling complex formation and internalization occur in lipid rafts of type I and type II cells.Eur J Immunol. 2004 Jul;34(7):1930-40. doi: 10.1002/eji.200324786. Eur J Immunol. 2004. PMID: 15214041
-
Rewinding the DISC.Arch Immunol Ther Exp (Warsz). 2008 Jan-Feb;56(1):9-14. doi: 10.1007/s00005-008-0002-9. Epub 2008 Feb 5. Arch Immunol Ther Exp (Warsz). 2008. PMID: 18250974 Review.
-
Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis.Biochem Soc Trans. 2004 Nov;32(Pt 5):679-81. doi: 10.1042/BST0320679. Biochem Soc Trans. 2004. PMID: 15493986 Review.
Cited by
-
Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice.PLoS One. 2013 Apr 12;8(4):e61380. doi: 10.1371/journal.pone.0061380. Print 2013. PLoS One. 2013. PMID: 23593476 Free PMC article.
-
Selective reduction in the sphingomyelin content of atherogenic lipoproteins inhibits their retention in murine aortas and the subsequent development of atherosclerosis.Arterioscler Thromb Vasc Biol. 2010 Nov;30(11):2114-20. doi: 10.1161/ATVBAHA.110.213363. Epub 2010 Sep 2. Arterioscler Thromb Vasc Biol. 2010. PMID: 20814016 Free PMC article.
-
γ-Tocotrienol induces apoptosis in pancreatic cancer cells by upregulation of ceramide synthesis and modulation of sphingolipid transport.BMC Cancer. 2018 May 16;18(1):564. doi: 10.1186/s12885-018-4462-y. BMC Cancer. 2018. PMID: 29769046 Free PMC article.
-
Imaging Mass Spectrometry Reveals Acyl-Chain- and Region-Specific Sphingolipid Metabolism in the Kidneys of Sphingomyelin Synthase 2-Deficient Mice.PLoS One. 2016 Mar 24;11(3):e0152191. doi: 10.1371/journal.pone.0152191. eCollection 2016. PLoS One. 2016. PMID: 27010944 Free PMC article.
-
Reducing plasma membrane sphingomyelin increases insulin sensitivity.Mol Cell Biol. 2011 Oct;31(20):4205-18. doi: 10.1128/MCB.05893-11. Epub 2011 Aug 15. Mol Cell Biol. 2011. PMID: 21844222 Free PMC article.
References
-
- Marsden, V.S., and A. Strasser. 2003. Control of apoptosis in the immune system: Bcl-2, BH-3 only proteins and more. Annu. Rev. Immunol. 21:71–105. - PubMed
-
- Wajant, H. 2002. The Fas signaling pathway: more than a paradigm. Science. 296:1635–1636. - PubMed
-
- Hengartner, M.O. 2000. The biochemistry of apoptosis. Nature. 407:770–776. - PubMed
-
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

