Dynamic assembly and sustained retention of 53BP1 at the sites of DNA damage are controlled by Mdc1/NFBD1
- PMID: 16009723
- PMCID: PMC2171401
- DOI: 10.1083/jcb.200503043
Dynamic assembly and sustained retention of 53BP1 at the sites of DNA damage are controlled by Mdc1/NFBD1
Abstract
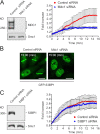
53BP1 is a key component of the genome surveillance network activated by DNA double strand breaks (DSBs). Despite its known accumulation at the DSB sites, the spatiotemporal aspects of 53BP1 interaction with DSBs and the role of other DSB regulators in this process remain unclear. Here, we used real-time microscopy to study the DSB-induced redistribution of 53BP1 in living cells. We show that within minutes after DNA damage, 53BP1 becomes progressively, yet transiently, immobilized around the DSB-flanking chromatin. Quantitative imaging of single cells revealed that the assembly of 53BP1 at DSBs significantly lagged behind Mdc1/NFBD1, another DSB-interacting checkpoint mediator. Furthermore, short interfering RNA-mediated ablation of Mdc1/NFBD1 drastically impaired 53BP1 redistribution to DSBs and triggered premature dissociation of 53BP1 from these regions. Collectively, these in vivo measurements identify Mdc1/NFBD1 as a key upstream determinant of 53BP1's interaction with DSBs from its dynamic assembly at the DSB sites through sustained retention within the DSB-flanking chromatin up to the recovery from the checkpoint.
Figures






References
-
- Celeste, A., O. Fernandez-Capetillo, M.J. Kruhlak, D.R. Pilch, D.W. Staudt, A. Lee, R.F. Bonner, W.M. Bonner, and A. Nussenzweig. 2003. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat. Cell Biol. 5:675–679. - PubMed
-
- D'Amours, D., and S.P. Jackson. 2002. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell Biol. 3:317–327. - PubMed
-
- DiTullio, R.A., Jr., T.A. Mochan, M. Venere, J. Bartkova, M. Sehested, J. Bartek, and T.D. Halazonetis. 2002. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat. Cell Biol. 4:998–1002. - PubMed
-
- Downs, J.A., S. Allard, O. Jobin-Robitaille, A. Javaheri, A. Auger, N. Bouchard, S.J. Kron, S.P. Jackson, and J. Cote. 2004. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol. Cell. 16:979–990. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

