Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung
- PMID: 16009933
- PMCID: PMC1177409
- DOI: 10.1073/pnas.0504520102
Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung
Abstract
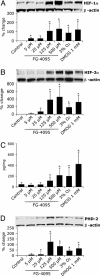
Preterm neonates with respiratory distress syndrome (RDS) often develop a chronic form of lung disease called bronchopulmonary dysplasia (BPD), characterized by decreased alveolar and vascular development. Ventilator treatment with supraphysiological O2 concentrations (hyperoxia) contribute to the development of BPD. Hyperoxia down-regulates and hypoxia up-regulates many angiogenic factors in the developing lung. We investigated whether angiogenic responses could be augmented through enhancement of hypoxia-inducible factors 1alpha and 2alpha (HIF-1alpha and -2alpha, respectively) via blockade of prolyl hydroxylase domain-containing proteins (HIF-PHDs) in human microvascular endothelial cells from developing and adult lung, in epithelial A549 cells, and in fetal baboon explants in relative or absolute hyperoxia. PHD inhibitor (FG-4095) and positive control dimethyloxaloylglycine (DMOG), selective and nonselective HIF-PHD inhibitors, respectively, enhanced HIF-1alpha and -2alpha, vascular endothelial growth factor (VEGF), and platelet-endothelial cell adhesion molecule 1 expression in vitro in 95% and 21% O2. Furthermore, VEGF receptor fms-like tyrosine kinase 1 (Flt-1) was elevated, whereas kinase insert domain-containing receptor/fetal liver kinase 1 (KDR) was diminished in endothelial, but not epithelial, cells. Intracellular Flt-1 and KDR locations were unchanged by PHD blockade. Like VEGF, FG-4095 and DMOG increased angiogenesis in vitro, both in 95% and 21% O2, an effect that could be blocked through either Flt-1 or KDR. Notably, FG-4095 was effective in stimulating HIFs and VEGF also in fetal baboon lung explants. FG-4095 or DMOG treatment appeared to stimulate the feedback loop promoting HIF degradation in that PHD-2 and/or -3, but not PHD-1, were enhanced. Through actions characterized above, FG-4095 could have desirable effects in enhancing lung growth in BPD.
Figures

References
-
- Wood, S. M., Gleadle, J. M., Pugh, C. W., Hankinson, O. & Ratcliffe, P. J. (1996) J. Biol. Chem. 271, 15117-15123. - PubMed
-
- Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., Kriegsheim, A., Hebestreit, H. F., Mukherji, M., Schofield, C. J., et al. (2001) Science 292, 468-472. - PubMed
-
- Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J. M., Lane, W. S. & Kaelin, W. G., Jr. (2001) Science 292, 464-468. - PubMed
-
- Epstein, A. C., Gleadle, J. M., McNeill, L. A., Hewitson, K. S., O'Rourke, J., Mole, D. R., Mukherji, M., Metzen, E., Wilson, M. I., Dhanda, A., et al. (2001) Cell 107, 43-54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

