Nutritional control of gene expression: how mammalian cells respond to amino acid limitation
- PMID: 16011459
- PMCID: PMC3600373
- DOI: 10.1146/annurev.nutr.24.012003.132145
Nutritional control of gene expression: how mammalian cells respond to amino acid limitation
Abstract
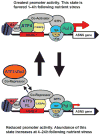
The amino acid response (AAR) pathway in mammalian cells is designed to detect and respond to amino acid deficiency. Limiting any essential amino acid initiates this signaling cascade, which leads to increased translation of a "master regulator," activating transcription factor (ATF) 4, and ultimately, to regulation of many steps along the pathway of DNA to RNA to protein. These regulated events include chromatin remodeling, RNA splicing, nuclear RNA export, mRNA stabilization, and translational control. Proteins that are increased in their expression as targets of the AAR pathway include membrane transporters, transcription factors from the basic region/leucine zipper (bZIP) superfamily, growth factors, and metabolic enzymes. Significant progress has been achieved in understanding the molecular mechanisms by which amino acids control the synthesis and turnover of mRNA and protein. Beyond gaining additional knowledge of these important regulatory pathways, further characterization of how these processes contribute to the pathology of various disease states represents an interesting aspect of future research in molecular nutrition.
Figures



References
-
- Abcouwer SF, Marjon PL, Loper RK, Vander Jagt DL. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest Ophthalmol Vis Sci. 2002;43:2791–98. - PubMed
-
- Abcouwer SF, Schwarz C, Meguid RA. Glutamine deprivation induces the expression of GADD45 and GADD153 primarily by mRNA stabilization. J Biol Chem. 1999;274:28645–51. - PubMed
-
- Adilakshmi T, Laine RO. Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death. J Biol Chem. 2002;277:4147–51. - PubMed
-
- Albrecht G, Mösch H-U, Hoffmann B, Reusser U, Braus GH. Monitoring the Gcn4 protein-mediated response in the yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:12696–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

