The retinoid anticancer signal: mechanisms of target gene regulation
- PMID: 16012519
- PMCID: PMC2361573
- DOI: 10.1038/sj.bjc.6602700
The retinoid anticancer signal: mechanisms of target gene regulation
Abstract
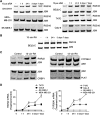
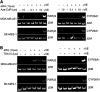
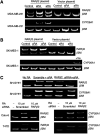
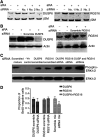
Retinoids induce growth arrest, differentiation, and cell death in many cancer cell types. One factor determining the sensitivity or resistance to the retinoid anticancer signal is the transcriptional response of retinoid-regulated target genes in cancer cells. We used cDNA microarray to identify 31 retinoid-regulated target genes shared by two retinoid-sensitive neuroblastoma cell lines, and then sought to determine the relevance of the target gene responses to the retinoid anticancer signal. The pattern of retinoid responsiveness for six of 13 target genes (RARbeta2, CYP26A1, CRBP1, RGS16, DUSP6, EGR1) correlated with phenotypic retinoid sensitivity, across a panel of retinoid-sensitive or -resistant lung and breast cancer cell lines. Retinoid treatment of MYCN transgenic mice bearing neuroblastoma altered the expression of five of nine target genes examined (RARbeta2, CYP26A1, CRBP1, DUSP6, PLAT) in neuroblastoma tumour tissue in vivo. In retinoid-sensitive neuroblastoma, lung and breast cancer cell lines, direct inhibition of retinoid-induced RARbeta2 expression blocked induction of only one of eight retinoid target genes (CYP26A1). DNA demethylation, histone acetylation, and exogenous overexpression of RARbeta2 partially restored retinoid-responsive CYP26A1 expression in RA-resistant MDA-MB-231 breast, but not SK-MES-1 lung, cancer cells. Combined, rather than individual, inhibition of DUSP6 and RGS16 was required to block retinoid-induced growth inhibition in neuroblastoma cells, through phosphorylation of extracellular-signal-regulated kinase. In conclusion, sensitivity to the retinoid anticancer signal is determined in part by the transcriptional response of key retinoid-regulated target genes, such as RARbeta2, DUSP6, and RGS16.
Figures
Similar articles
-
Retinoic acid and TGF-β signalling cooperate to overcome MYCN-induced retinoid resistance.Genome Med. 2017 Feb 10;9(1):15. doi: 10.1186/s13073-017-0407-3. Genome Med. 2017. PMID: 28187790 Free PMC article.
-
The estrogen-responsive B box protein (EBBP) restores retinoid sensitivity in retinoid-resistant cancer cells via effects on histone acetylation.Cancer Lett. 2009 May 8;277(1):82-90. doi: 10.1016/j.canlet.2008.11.030. Epub 2009 Jan 14. Cancer Lett. 2009. PMID: 19147277
-
Growth inhibitory retinoid effects after recruitment of retinoid X receptor beta to the retinoic acid receptor beta promoter.Int J Cancer. 2003 Jul 20;105(6):856-67. doi: 10.1002/ijc.11153. Int J Cancer. 2003. PMID: 12767074
-
Retinoid, retinoic acid receptor beta and breast cancer.Breast Cancer Res Treat. 2002 Nov;76(2):167-73. doi: 10.1023/a:1020576606004. Breast Cancer Res Treat. 2002. PMID: 12452454 Review.
-
Complexity, retinoid-responsive gene networks, and bladder carcinogenesis.Adv Exp Med Biol. 1999;462:449-67. doi: 10.1007/978-1-4615-4737-2_35. Adv Exp Med Biol. 1999. PMID: 10599447 Review.
Cited by
-
In Vitro Transfection of Up-Regulated Genes Identified in Favorable-Outcome Neuroblastoma into Cell Lines.Cells. 2022 Oct 10;11(19):3171. doi: 10.3390/cells11193171. Cells. 2022. PMID: 36231133 Free PMC article.
-
Studies on Multifunctional Effect of All-Trans Retinoic Acid (ATRA) on Matrix Metalloproteinase-2 (MMP-2) and Its Regulatory Molecules in Human Breast Cancer Cells (MCF-7).J Oncol. 2009;2009:627840. doi: 10.1155/2009/627840. Epub 2009 Jul 19. J Oncol. 2009. PMID: 19636436 Free PMC article.
-
Reciprocal regulation of extracellular signal regulated kinase 1/2 and mitogen activated protein kinase phosphatase-3.Toxicol Appl Pharmacol. 2008 Nov 1;232(3):408-17. doi: 10.1016/j.taap.2008.08.007. Epub 2008 Aug 15. Toxicol Appl Pharmacol. 2008. PMID: 18771677 Free PMC article.
-
Maternal consumption of canola oil suppressed mammary gland tumorigenesis in C3(1) TAg mice offspring.BMC Cancer. 2010 Mar 6;10:81. doi: 10.1186/1471-2407-10-81. BMC Cancer. 2010. PMID: 20205934 Free PMC article.
-
Diet components can suppress inflammation and reduce cancer risk.Nutr Res Pract. 2014 Jun;8(3):233-40. doi: 10.4162/nrp.2014.8.3.233. Epub 2014 May 15. Nutr Res Pract. 2014. PMID: 24944766 Free PMC article. Review.
References
-
- Altucci L, Gronemeyer H (2001) The promise of retinoids to fight against cancer. Nat Rev Cancer 1: 181–193 - PubMed
-
- Balmer JE, Blomhoff R (2002) Gene expression regulation by retinoic acid. J Lipid Res 43: 1773–1808 - PubMed
-
- Becker EW (2004) Relevance of the kinetic equilibrium of forces to the control of the cell cycle by Ras proteins. Biol Chem 385: 41–47 - PubMed
-
- Bordow SB, Norris MD, Haber PS, Marshall GM, Haber M (1998) Prognostic significance of MYCN oncogene expression in childhood neuroblastoma. J Clin Oncol 16: 3286–3294 - PubMed
-
- Boussioutas A, Li H, Liu J, Waring P, Lade S, Holloway AJ, Taupin D, Gorringe K, Haviv I, Desmond PV, Bowtell DD (2003) Distinctive patterns of gene expression in premalignant gastric mucosa and gastric cancer. Cancer Res 63: 2569–2577 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

