Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver
- PMID: 16012954
- PMCID: PMC1821080
- DOI: 10.1053/j.gastro.2005.04.013
Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver
Abstract
Background & aims: Wnt/beta-catenin activation is observed in normal liver development, regeneration, and liver cancer. Our aim was to elucidate the regulation and mechanism of this pathway in liver.
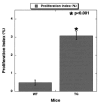
Methods: We report the generation and characterization of liver-specific nonmutated beta-catenin-overexpressing transgenic mice. Transgenic livers were examined for their morphology and phenotype by histology, proliferation, apoptosis, and microarray analysis.
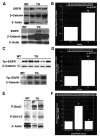
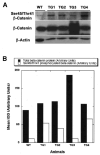
Results: Transgenic livers displayed a significant increase in cytoplasmic, membranous, and nuclear beta-catenin in hepatocytes as compared with their wild-type littermates, which display a predominant membranous localization only. A 15%-20% increase in the liver weight-body weight ratio was evident in transgenic mice secondary to increased hepatocyte proliferation. Microarray analysis showed differential expression of approximately 400 genes in the transgenic livers. Epidermal growth factor receptor RNA and protein and increased levels of activated epidermal growth factor receptor and Stat3 were observed in the transgenic livers. Epidermal growth factor receptor promoter analysis showed a T-cell factor-binding site, and subsequent reporter assay confirmed epidermal growth factor receptor activation in response to Wnt-3A treatment that was abrogated by frizzled related protein 1, a known Wnt antagonist. Epidermal growth factor receptor inhibition successfully decreased liver size in transgenic mice. Next, 7 of 10 hepatoblastomas displayed simultaneous beta-catenin and epidermal growth factor receptor up-regulation, thus suggesting a strong relationship between these 2 proteins in tumors.
Conclusions: beta-Catenin transgenic mice show an in vivo hepatotrophic effect secondary to increased basal hepatocyte proliferation. Epidermal growth factor receptor seems to be a direct target of the pathway, and epidermal growth factor receptor activation might contribute toward some mitogenic effects of increased beta-catenin in liver: epidermal growth factor receptor inhibition might be useful in such states.
Conflict of interest statement
George K. Michalopoulos is a consultant for Kytaron, Corp. There are no other conflicts of interest to be disclosed.
Figures





Similar articles
-
Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer.J Hepatol. 2005 Jun;42(6):842-9. doi: 10.1016/j.jhep.2005.01.029. Epub 2005 Apr 8. J Hepatol. 2005. PMID: 15885355
-
Elevated expression of Wnt antagonists is a common event in hepatoblastomas.Clin Cancer Res. 2005 Jun 15;11(12):4295-304. doi: 10.1158/1078-0432.CCR-04-1162. Clin Cancer Res. 2005. PMID: 15958610
-
Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin.Cancer Res. 2001 Apr 15;61(8):3245-9. Cancer Res. 2001. PMID: 11309273
-
Overview of the molecular biology of hepatocellular neoplasms and hepatoblastomas of the mouse liver.Toxicol Pathol. 2005;33(1):175-80. doi: 10.1080/01926230590522130. Toxicol Pathol. 2005. PMID: 15805069 Review.
-
Wnt/β-catenin signaling as a useful therapeutic target in hepatoblastoma.Biosci Rep. 2019 Sep 24;39(9):BSR20192466. doi: 10.1042/BSR20192466. Print 2019 Sep 30. Biosci Rep. 2019. PMID: 31511432 Free PMC article. Review.
Cited by
-
β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis.Gastroenterology. 2015 Jun;148(7):1294-310. doi: 10.1053/j.gastro.2015.02.056. Epub 2015 Mar 5. Gastroenterology. 2015. PMID: 25747274 Free PMC article. Review.
-
Wnt signaling in gut organogenesis.Organogenesis. 2008 Apr;4(2):87-91. doi: 10.4161/org.4.2.5854. Organogenesis. 2008. PMID: 19279719 Free PMC article.
-
Hepatic cancer stem cells and drug resistance: Relevance in targeted therapies for hepatocellular carcinoma.World J Hepatol. 2010 Mar 27;2(3):114-26. doi: 10.4254/wjh.v2.i3.114. World J Hepatol. 2010. PMID: 21160982 Free PMC article.
-
β-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma.Cancer Res. 2014 Aug 15;74(16):4515-25. doi: 10.1158/0008-5472.CAN-13-3275. Epub 2014 May 21. Cancer Res. 2014. PMID: 24848510 Free PMC article.
-
Hepatocyte Wnts Are Dispensable During Diethylnitrosamine and Carbon Tetrachloride-Induced Injury and Hepatocellular Cancer.Gene Expr. 2018 Aug 22;18(3):209-219. doi: 10.3727/105221618X15205148413587. Epub 2018 Mar 8. Gene Expr. 2018. PMID: 29519268 Free PMC article.
References
-
- Pennisi E. How a growth control path takes a wrong turn to cancer (published erratum appears in Science 1998;281:1809) Science. 1998;281:1438–1439. 1441. - PubMed
-
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. - PubMed
-
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. - PubMed
-
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

