Attempts to characterize the NBD heterodimer of MRP1: transient complex formation involves Gly771 of the ABC signature sequence but does not enhance the intrinsic ATPase activity
- PMID: 16014004
- PMCID: PMC1276949
- DOI: 10.1042/BJ20050897
Attempts to characterize the NBD heterodimer of MRP1: transient complex formation involves Gly771 of the ABC signature sequence but does not enhance the intrinsic ATPase activity
Abstract
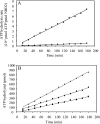
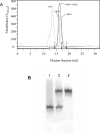
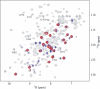
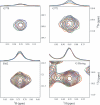
MRP1 (multidrug-resistance-associated protein 1; also known as ABCC1) is a member of the human ABC (ATP-binding cassette) transporter superfamily that confers cell resistance to chemotherapeutic agents. Considering the structural and functional similarities to the other ABC proteins, the interaction between its two NBDs (nucleotide-binding domains), NBD1 (N-terminal NBD) and NBD2 (C-terminal NBD), is proposed to be essential for the regulation of the ATP-binding/ATP-hydrolysis cycle of MRP1. We were interested in the ability of recombinant NBD1 and NBD2 to interact with each other and to influence ATPase activity. We purified NBD1 (Asn642-Ser871) and NBD2 (Ser1286-Val1531) as soluble monomers under native conditions. We measured extremely low intrinsic ATPase activity of NBD1 (10(-5) s(-1)) and NBD2 (6x10(-6) s(-1)) and no increase in the ATP-hydrolysis rate could be detected in an NBD1+NBD2 mixture, with concentrations up to 200 microM. Despite the fact that both monomers bind ATP, no stable NBD1.NBD2 heterodimer could be isolated by gel-filtration chromatography or native-PAGE, but we observed some significant modifications of the heteronuclear single-quantum correlation NMR spectrum of 15N-NBD1 in the presence of NBD2. This apparent NBD1.NBD2 interaction only occurred in the presence of Mg2+ and ATP. Partial sequential assignment of the NBD1 backbone resonances shows that residue Gly771 of the LSGGQ sequence is involved in NBD1.NBD2 complex formation. This is the first NMR observation of a direct interaction between the ABC signature and the opposite NBD. Our study also reveals that the NBD1.NBD2 heterodimer of MRP1 is a transient complex. This labile interaction is not sufficient to induce an ATPase co-operativity of the NBDs and suggests that other structures are required for the ATPase activation mechanism.
Figures


References
-
- Holland B., Cole S. P., Kuchler K., Higgins C. F., editors. London: Academic Press; 2003. ABC Proteins from Bacteria to M.
-
- Hipfner D. R., Deeley R. G., Cole S. P. Structural, mechanistic and clinical aspects of MRP1. Biochim. Biophys. Acta. 1999;1461:359–376. - PubMed
-
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. - PubMed
-
- Cole S. P., Deeley R. G. Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. BioEssays. 1998;20:931–940. - PubMed
-
- Leslie E. M., Deeley R. G., Cole S. P. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167:3–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

