Cystine and glutamate transport in renal epithelial cells transfected with human system x(-) (c)
- PMID: 16014042
- PMCID: PMC2409290
- DOI: 10.1111/j.1523-1755.2005.00443.x
Cystine and glutamate transport in renal epithelial cells transfected with human system x(-) (c)
Abstract
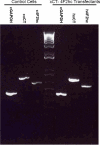
Background: System x(-) (c) is a heterodimeric transporter, comprised of a light chain, xCT, and heavy chain, 4F2hc, which mediates the sodium-independent exchange of cystine and glutamate at the plasma membrane. In the current study we tested the hypothesis that stable transfection of Madin-Darby canine kidney (MDCK) cells with human xCT and 4F2hc results in the expression of functional system x(-) (c).
Methods: MDCK cells were transfected stably with human clones for xCT and 4F2hc. Analyses of time- and temperature-dependence, saturation kinetics, and substrate specificity of l-cystine and l-glutamate transport were carried out in control and xCT-4F2hc-transfected MDCK cells. We also measured the uptake of l-cystine in Xenopus oocytes expressing human xCT and/or 4F2hc or xCT and/or rBAT (a heavy chain homologous to 4F2hc).
Results: All of the different sets of data revealed that transport of l-cystine and l-glutamate increased significantly (twofold to threefold) in the MDCK cells subsequent to transfection with xCT-4F2hc. Moreover, uptake of l-cystine also increased (about tenfold) in Xenopus oocytes expressing hxCT and h4F2hc. Biochemical analyses of l-cystine uptake in oocytes verified our findings in the transfected MDCK cells. Interestingly, in oocytes injected with rBAT with or without xCT, uptake of l-cystine was significantly greater than that in water-injected oocytes.
Conclusion: Our findings indicate that stable transfection of MDCK cells with xCT and 4F2hc results in a cell-line expressing a functional system x(-) (c) transporter that can utilize l-cystine and l-glutamate as substrates. This study apparently represents the first stable transfection of a mammalian cell line with system x(-) (c).
Figures








References
-
- Aslamkhan AG, Han YH, Yang XP, et al. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol Pharmacol. 2003;63:590–596. - PubMed
-
- Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984;779:289–306. - PubMed
-
- Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. - PubMed
-
- Bannai S, Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol. 1982;112:265–272. - PubMed
-
- Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255:2372–2376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

