Use of a novel cell-based fusion reporter assay to explore the host range of human respiratory syncytial virus F protein
- PMID: 16014172
- PMCID: PMC1190219
- DOI: 10.1186/1743-422X-2-54
Use of a novel cell-based fusion reporter assay to explore the host range of human respiratory syncytial virus F protein
Abstract
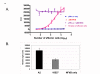
Human respiratory syncytial virus (HRSV) is an important respiratory pathogen primarily affecting infants, young children, transplant recipients and the elderly. The F protein is the only virion envelope protein necessary and sufficient for virus replication and fusion of the viral envelope membrane with the target host cell. During natural infection, HRSV replication is limited to respiratory epithelial cells with disseminated infection rarely, if ever, occurring even in immunocompromised patients. However, in vitro infection of multiple human and non-human cell types other than those of pulmonary tract origin has been reported. To better define host cell surface molecules that mediate viral entry and dissect the factors controlling permissivity for HRSV, we explored the host range of HRSV F protein mediated fusion. Using a novel recombinant reporter gene based fusion assay, HRSV F protein was shown to mediate fusion with cells derived from a wide range of vertebrate species including human, feline, equine, canine, bat, rodent, avian, porcine and even amphibian (Xenopus). That finding was extended using a recombinant HRSV engineered to express green fluorescent protein (GFP), to confirm that viral mRNA expression is limited in several cell types. These findings suggest that HRSV F protein interacts with either highly conserved host cell surface molecules or can use multiple mechanisms to enter cells, and that the primary determinants of HRSV host range are at steps post-entry.
Figures


References
-
- Ison MGHFG. Viral infections in immunocompromised patients:what's new with respiratory viruses? Curr Opin Infect Dis. 2002;15:355–367. - PubMed
-
- Collins PLCRMMBR. Respiratory syncytial virus. In: D.M. Knipe HPM, editor. Fields Virology. Vol. 1. Philadelphia, Lippincott, Williams, and Wilkins; 2001. pp. 1443–1485.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

