Suppression of myasthenogenic responses of a T cell line by a dual altered peptide ligand by induction of CD4+CD25+ regulatory cells
- PMID: 16014414
- PMCID: PMC1177416
- DOI: 10.1073/pnas.0504578102
Suppression of myasthenogenic responses of a T cell line by a dual altered peptide ligand by induction of CD4+CD25+ regulatory cells
Erratum in
- Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12288
Abstract
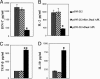
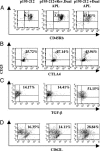
Myasthenia gravis is a T cell-dependent, antibody-mediated autoimmune disease. A dual altered peptide ligand (APL) that is composed of the tandemly arranged two single amino acid analogs of two myasthenogenic peptides, p195-212 and p259-271, was demonstrated to down-regulate in vitro and in vivo myasthenia gravis-associated autoreactive responses. The aims of this study were to demonstrate the suppressive properties and to elucidate the mechanism of action of the dual APL on a T cell line specific to the myasthenogenic peptide p195-212. We demonstrate here that incubation of cells of the line with the dual APL resulted in the inhibition of proliferation and secretion of IL-2 and IFN-gamma triggered by p195-212. In contrast, secretion of TGF-beta and IL-10 was upregulated. The dual APL induced the generation of CD4+CD25+ cells that were characterized by the expression of CD45Rb(low), cytotoxic T lymphocyte-associated antigen-4, TGF-beta, CD62L, Foxp3, and neuropilin. In addition, the dual APL-treated cells were capable of inhibiting the proliferation response of the line when the two sets of cells were cocultured. The role of CD4+CD25+ cells was further confirmed by demonstrating that the suppression was abrogated by blocking/neutralization of CD25. Thus, the dual APL acts by inducing the formation of CD4+CD25+ regulatory cells. By using a T cell line, we could show that the immunosuppressive CD4+CD25+ cells were indeed induced by the dual APL and are not part of the naturally occurring regulatory cells.
Figures



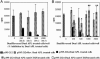
Similar articles
-
A dual altered peptide ligand down-regulates myasthenogenic T cell responses by up-regulating CD25- and CTLA-4-expressing CD4+ T cells.Proc Natl Acad Sci U S A. 2003 May 27;100(11):6676-81. doi: 10.1073/pnas.1131898100. Epub 2003 May 12. Proc Natl Acad Sci U S A. 2003. PMID: 12743364 Free PMC article.
-
A dual altered peptide ligand inhibits myasthenia gravis associated responses by inducing phosphorylated extracellular-regulated kinase 1,2 that upregulates CD4+CD25+Foxp3+ cells.Scand J Immunol. 2007 Jun;65(6):567-76. doi: 10.1111/j.1365-3083.2007.01940.x. Scand J Immunol. 2007. PMID: 17523950
-
The role of CD8+CD28 regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand.Proc Natl Acad Sci U S A. 2007 Oct 30;104(44):17459-64. doi: 10.1073/pnas.0708577104. Epub 2007 Oct 23. Proc Natl Acad Sci U S A. 2007. PMID: 17956982 Free PMC article.
-
Therapeutic vaccines in autoimmunity.Proc Natl Acad Sci U S A. 2004 Oct 5;101 Suppl 2(Suppl 2):14586-92. doi: 10.1073/pnas.0404826101. Epub 2004 Aug 12. Proc Natl Acad Sci U S A. 2004. PMID: 15308777 Free PMC article. Review.
-
Modification of human T-cell responses by altered peptide ligands: a new approach to antigen-specific modification.Intern Med. 1998 Oct;37(10):804-17. doi: 10.2169/internalmedicine.37.804. Intern Med. 1998. PMID: 9840700 Review.
Cited by
-
Two lysines in the forkhead domain of foxp3 are key to T regulatory cell function.PLoS One. 2012;7(1):e29035. doi: 10.1371/journal.pone.0029035. Epub 2012 Jan 11. PLoS One. 2012. PMID: 22247766 Free PMC article.
-
Natural Tregs, CD4+CD25+ inhibitory hybridomas, and their cell contact dependent suppression.Immunol Res. 2007;39(1-3):62-78. doi: 10.1007/s12026-007-0064-5. Immunol Res. 2007. PMID: 17917056
-
The mutant leucine-zipper domain impairs both dimerization and suppressive function of Foxp3 in T cells.Proc Natl Acad Sci U S A. 2006 Jun 20;103(25):9631-6. doi: 10.1073/pnas.0600225103. Epub 2006 Jun 12. Proc Natl Acad Sci U S A. 2006. PMID: 16769892 Free PMC article.
-
A peptide derived from HSP60 reduces proinflammatory cytokines and soluble mediators: a therapeutic approach to inflammation.Front Immunol. 2023 Apr 28;14:1162739. doi: 10.3389/fimmu.2023.1162739. eCollection 2023. Front Immunol. 2023. PMID: 37187739 Free PMC article. Review.
-
APL-1, an altered peptide ligand derived from heat-shock protein, alone or combined with methotrexate attenuates murine collagen-induced arthritis.Clin Exp Med. 2017 May;17(2):209-216. doi: 10.1007/s10238-016-0412-7. Epub 2016 May 9. Clin Exp Med. 2017. PMID: 27160252
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

