Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo
- PMID: 16014565
- PMCID: PMC1895340
- DOI: 10.1182/blood-2005-05-1864
Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo
Abstract
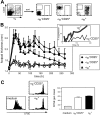
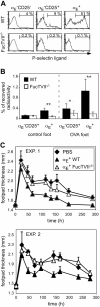
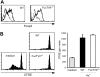
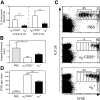
Regulatory T cells (Tregs) play a fundamental role in the suppression of different immune responses; however, compartments at which they exert suppressive functions in vivo are unknown. Although many groups have described the presence of Tregs within inflammatory sites, it has not been shown that inflamed tissues are, indeed, the sites of active suppression of ongoing immune reactions. Here, by using alpha(E)+ effector/memory-like Tregs from fucosyltransferase VII-deficient animals, which lack E/P-selectin ligands and fail to migrate into inflamed sites, we analyzed the functional importance of appropriate Treg localization for in vivo suppressive capacity in an inflammation model. Lack of suppression by Tregs deficient in E/P-selectin ligands demonstrates that immigration into inflamed sites is a prerequisite for the resolution of inflammatory reactions in vivo because these selectin ligands merely regulate entry into inflamed tissues. In contrast, control of proliferation of naive CD4+ T cells during the induction phase of the immune response is more efficiently exerted by the naive-like alpha(E)-CD25+ Treg subset preferentially recirculating through lymph nodes when compared with its inflammation-seeking counterpart. Together, these findings provide the first conclusive evidence that appropriate localization is crucial for in vivo activity of Tregs and might have significant implications for anti-inflammatory therapies targeting recruitment mechanisms.
Figures

References
-
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22: 531-562. - PubMed
-
- Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10: 1969-1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

