Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells
- PMID: 16014609
- PMCID: PMC1237057
- DOI: 10.1091/mbc.e05-01-0061
Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells
Erratum in
- Mol Biol Cell. 2005 Dec;16(12):5901
Abstract
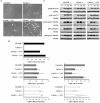
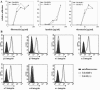
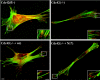
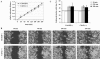
Cdc42 is a small GTPase involved in the regulation of the cytoskeleton and cell polarity. To test whether Cdc42 has an essential role in the formation of filopodia or directed cell migration, we generated Cdc42-deficient fibroblastoid cells by conditional gene inactivation. We report here that loss of Cdc42 did not affect filopodium or lamellipodium formation and had no significant influence on the speed of directed migration nor on mitosis. Cdc42-deficient cells displayed a more elongated cell shape and had a reduced area. Furthermore, directionality during migration and reorientation of the Golgi apparatus into the direction of migration was decreased. However, expression of dominant negative Cdc42 in Cdc42-null cells resulted in strongly reduced directed migration, severely reduced single cell directionality, and complete loss of Golgi polarization and of directionality of protrusion formation toward the wound, as well as membrane blebbing. Thus, our data show that besides Cdc42 additional GTPases of the Rho-family, which share GEFs with Cdc42, are involved in the establishment and maintenance of cell polarity during directed migration.
Figures




References
-
- Abe, T., Kato, M., Miki, H., Takenawa, T., and Endo, T. (2003). Small GTPase Tc10 and its homologue RhoT induce N-WASP-mediated long process formation and neurite outgrowth. J. Cell Sci. 116, 155–168. - PubMed
-
- Bokoch, G. M. (2003). Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

