Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats
- PMID: 16014703
- PMCID: PMC1262647
- DOI: 10.1152/ajpcell.00329.2004
Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats
Abstract
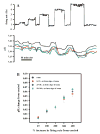
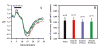
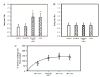
Cardiorespiratory control is mediated in part by central chemosensitive neurons that respond to increased CO(2) (hypercapnia). Activation of these neurons is thought to involve hypercapnia-induced decreases in intracellular pH (pH(i)). All previous measurements of hypercapnia-induced pH(i) changes in chemosensitive neurons have been obtained from the soma, but chemosensitive signaling could be initiated in the dendrites of these neurons. In this study, membrane potential (V(m)) and pH(i) were measured simultaneously in chemosensitive locus coeruleus (LC) neurons from neonatal rat brain stem slices using whole cell pipettes and the pH-sensitive fluorescent dye pyranine. We measured pH(i) from the soma as well as from primary dendrites to a distance 160 mum from the edge of the soma. Hypercapnia [15% CO(2), external pH (pH(o)) 7.00; control, 5% CO(2), pH(o) 7.45] resulted in an acidification of similar magnitude in dendrites and soma ( approximately 0.26 pH unit), but acidification was faster in the more distal regions of the dendrites. Neither the dendrites nor the soma exhibited pH(i) recovery during hypercapnia-induced acidification; but both regions contained pH-regulating transporters, because they exhibited pH(i) recovery from an NH(4)Cl prepulse-induced acidification (at constant pH(o) 7.45). Exposure of a portion of the dendrites to hypercapnic solution did not increase the firing rate, but exposing the soma to hypercapnic solution resulted in a near-maximal increase in firing rate. These data show that while the pH(i) response to hypercapnia is similar in the dendrites and soma, somatic exposure to hypercapnia plays a major role in the activation of chemosensitive LC neurons from neonatal rats.
Figures






Similar articles
-
Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones.J Physiol. 2002 Jun 1;541(Pt 2):493-509. doi: 10.1113/jphysiol.2001.014142. J Physiol. 2002. PMID: 12042354 Free PMC article.
-
pH regulating transporters in neurons from various chemosensitive brainstem regions in neonatal rats.Am J Physiol Regul Integr Comp Physiol. 2009 Nov;297(5):R1409-20. doi: 10.1152/ajpregu.91038.2008. Epub 2009 Aug 26. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19710385 Free PMC article.
-
The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH.Adv Exp Med Biol. 2008;605:333-7. doi: 10.1007/978-0-387-73693-8_58. Adv Exp Med Biol. 2008. PMID: 18085295
-
Intracellular pH regulation of neurons in chemosensitive and nonchemosensitive areas of brain slices.Respir Physiol. 2001 Dec;129(1-2):37-56. doi: 10.1016/s0034-5687(01)00281-x. Respir Physiol. 2001. PMID: 11738645 Review.
-
Role of Astrocytes in Central Respiratory Chemoreception.Adv Exp Med Biol. 2016;949:109-145. doi: 10.1007/978-3-319-40764-7_6. Adv Exp Med Biol. 2016. PMID: 27714687 Review.
Cited by
-
The cellular building blocks of breathing.Compr Physiol. 2012 Oct;2(4):2683-731. doi: 10.1002/cphy.c110033. Compr Physiol. 2012. PMID: 23720262 Free PMC article. Review.
-
A HCO(3)(-)-dependent mechanism involving soluble adenylyl cyclase for the activation of Ca²⁺ currents in locus coeruleus neurons.Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2569-78. doi: 10.1016/j.bbadis.2014.07.027. Epub 2014 Aug 1. Biochim Biophys Acta. 2014. PMID: 25092170 Free PMC article.
-
Sudden Unexpected Death in Epilepsy: Central Respiratory Chemoreception.Int J Mol Sci. 2025 Feb 13;26(4):1598. doi: 10.3390/ijms26041598. Int J Mol Sci. 2025. PMID: 40004062 Free PMC article. Review.
-
Negative shift in the glycine reversal potential mediated by a Ca2+- and pH-dependent mechanism in interneurons.J Neurosci. 2009 Sep 16;29(37):11495-510. doi: 10.1523/JNEUROSCI.1086-09.2009. J Neurosci. 2009. PMID: 19759298 Free PMC article.
-
Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin.J Appl Physiol (1985). 2010 May;108(5):1417-24. doi: 10.1152/japplphysiol.01261.2009. Epub 2010 Feb 4. J Appl Physiol (1985). 2010. PMID: 20133433 Free PMC article. Review.
References
-
- Andrzejewski M, Mückenhoff K, Scheid P, Ballantyne D. Synchronized rhythms in chemosensitive neurons of the locus coeruleus in the absence of chemical synaptic transmission. Resp Physiol. 2001;129:123–140. - PubMed
-
- Ballantyne D, Scheid P. Central chemosensitivity of respiration: a brief overview. Resp Physiol. 2001;129:5–12. - PubMed
-
- Ballanyi K, Mückenhoff K, Bellingham MC, Okada Y, Scheid P, Richter DW. Activity-related pH changes in respiratory neurones and glial cells of cats. Neuroreport. 1994;6:33–36. - PubMed
-
- Boron WF. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol Cell Physiol. 1977;233:C61–C73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

