History-dependent multiple-time-scale dynamics in a single-neuron model
- PMID: 16014709
- PMCID: PMC6725418
- DOI: 10.1523/JNEUROSCI.0763-05.2005
History-dependent multiple-time-scale dynamics in a single-neuron model
Abstract
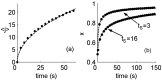
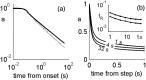
History-dependent characteristic time scales in dynamics have been observed at several levels of organization in neural systems. Such dynamics can provide powerful means for computation and memory. At the level of the single neuron, several microscopic mechanisms, including ion channel kinetics, can support multiple-time-scale dynamics. How the temporally complex channel kinetics gives rise to dynamical properties of the neuron is not well understood. Here, we construct a model that captures some features of the connection between these two levels of organization. The model neuron exhibits history-dependent multiple-time-scale dynamics in several effects: first, after stimulation, the recovery time scale is related to the stimulation duration by a power-law scaling; second, temporal patterns of neural activity in response to ongoing stimulation are modulated over time; finally, the characteristic time scale for adaptation after a step change in stimulus depends on the duration of the preceding stimulus. All these effects have been observed experimentally and are not explained by current single-neuron models. The model neuron here presented is composed of an ensemble of ion channels that can wander in a large pool of degenerate inactive states and thus exhibits multiple-time-scale dynamics at the molecular level. Channel inactivation rate depends on recent neural activity, which in turn depends through modulations of the neural response function on the fraction of active channels. This construction produces a model that robustly exhibits nonexponential history-dependent dynamics, in qualitative agreement with experimental results.
Figures

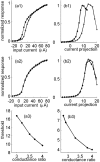

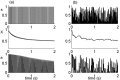
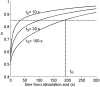
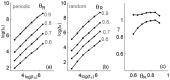


Similar articles
-
Information transmission and recovery in neural communications channels.Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000 Nov;62(5 Pt B):7111-22. doi: 10.1103/physreve.62.7111. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000. PMID: 11102068
-
Biophysically interpretable inference of single neuron dynamics.J Comput Neurosci. 2019 Aug;47(1):61-76. doi: 10.1007/s10827-019-00723-7. Epub 2019 Aug 29. J Comput Neurosci. 2019. PMID: 31468241
-
Single neuron computation: from dynamical system to feature detector.Neural Comput. 2007 Dec;19(12):3133-72. doi: 10.1162/neco.2007.19.12.3133. Neural Comput. 2007. PMID: 17970648
-
The use of automated parameter searches to improve ion channel kinetics for neural modeling.J Comput Neurosci. 2011 Oct;31(2):329-46. doi: 10.1007/s10827-010-0312-x. Epub 2011 Jan 18. J Comput Neurosci. 2011. PMID: 21243419
-
A spiking neuron model for binocular rivalry.J Comput Neurosci. 2002 Jan-Feb;12(1):39-53. doi: 10.1023/a:1014942129705. J Comput Neurosci. 2002. PMID: 11932559 Review.
Cited by
-
Distinct effects of brief and prolonged adaptation on orientation tuning in primary visual cortex.J Neurosci. 2013 Jan 9;33(2):532-43. doi: 10.1523/JNEUROSCI.3345-12.2013. J Neurosci. 2013. PMID: 23303933 Free PMC article.
-
Interpreting neurodynamics: concepts and facts.Cogn Neurodyn. 2008 Dec;2(4):297-318. doi: 10.1007/s11571-008-9067-8. Epub 2008 Oct 15. Cogn Neurodyn. 2008. PMID: 19003452 Free PMC article.
-
Fractional order memcapacitive neuromorphic elements reproduce and predict neuronal function.Sci Rep. 2024 Mar 9;14(1):5817. doi: 10.1038/s41598-024-55784-1. Sci Rep. 2024. PMID: 38461365 Free PMC article.
-
Temporal whitening by power-law adaptation in neocortical neurons.Nat Neurosci. 2013 Jul;16(7):942-8. doi: 10.1038/nn.3431. Epub 2013 Jun 9. Nat Neurosci. 2013. PMID: 23749146
-
Beyond faithful conduction: short-term dynamics, neuromodulation, and long-term regulation of spike propagation in the axon.Prog Neurobiol. 2011 Sep 1;94(4):307-46. doi: 10.1016/j.pneurobio.2011.06.001. Epub 2011 Jun 17. Prog Neurobiol. 2011. PMID: 21708220 Free PMC article. Review.
References
-
- Anderson JR (1995) Cognitive psychology and its implications. New York: Freeman.
-
- Bassingthwaighte JB, Liebovitch LS, West BJ (1994) Fractal physiology. New York: Oxford UP.
-
- Brenner N, de Ruyter van Steveninck RR, Bialek W (2000) Adaptive rescaling maximizes information transmission. Neuron 29: 695-702. - PubMed
-
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ (2003) Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron 39: 793-806. - PubMed
-
- de Ruyter van Steveninck RR, Zaagman WH, Masterbroek HAK (1986) Adaptation of transient responses of a movement-sensitive neuron in the visual system of the blowfly C. erythrocephala Biol Cybern 54: 223-236.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
