Modeling cooperative volume signaling in a plexus of nitric-oxide-synthase-expressing neurons
- PMID: 16014713
- PMCID: PMC6725429
- DOI: 10.1523/JNEUROSCI.1264-05.2005
Modeling cooperative volume signaling in a plexus of nitric-oxide-synthase-expressing neurons
Abstract
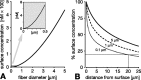
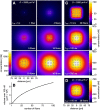
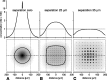
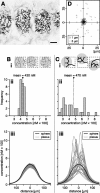
In vertebrate and invertebrate brains, nitric oxide (NO) synthase (NOS) is frequently expressed in extensive meshworks (plexuses) of exceedingly fine fibers. In this paper, we investigate the functional implications of this morphology by modeling NO diffusion in fiber systems of varying fineness and dispersal. Because size severely limits the signaling ability of an NO-producing fiber, the predominance of fine fibers seems paradoxical. Our modeling reveals, however, that cooperation between many fibers of low individual efficacy can generate an extensive and strong volume signal. Importantly, the signal produced by such a system of cooperating dispersed fibers is significantly more homogeneous in both space and time than that produced by fewer larger sources. Signals generated by plexuses of fine fibers are also better centered on the active region and less dependent on their particular branching morphology. We conclude that an ultrafine plexus is configured to target a volume of the brain with a homogeneous volume signal. Moreover, by translating only persistent regional activity into an effective NO volume signal, dispersed sources integrate neural activity over both space and time. In the mammalian cerebral cortex, for example, the NOS plexus would preferentially translate persistent regional increases in neural activity into a signal that targets blood vessels residing in the same region of the cortex, resulting in an increased regional blood flow. We propose that the fineness-dependent properties of volume signals may in part account for the presence of similar NOS plexus morphologies in distantly related animals.
Figures




Similar articles
-
Three-dimensional distribution of NO sources in a primary mechanosensory integration center in the locust and its implications for volume signaling.J Comp Neurol. 2010 Aug 1;518(15):2903-16. doi: 10.1002/cne.22396. J Comp Neurol. 2010. PMID: 20533352
-
Nitric oxide synthase in the thoracic ganglia of the locust: distribution in the neuropiles and morphology of neurones.J Comp Neurol. 1998 Jun 1;395(2):217-30. doi: 10.1002/(sici)1096-9861(19980601)395:2<217::aid-cne6>3.0.co;2-5. J Comp Neurol. 1998. PMID: 9603374
-
Nitrergic neurons make synapses on dual-input dendritic spines of neurons in the cerebral cortex and the striatum of the rat: implication for a postsynaptic action of nitric oxide.Neuroscience. 2000;99(4):627-42. doi: 10.1016/s0306-4522(00)00227-x. Neuroscience. 2000. PMID: 10974426
-
Traumatic injury of the spinal cord and nitric oxide.Prog Brain Res. 2007;161:171-83. doi: 10.1016/S0079-6123(06)61011-X. Prog Brain Res. 2007. PMID: 17618976 Review.
-
Distribution pattern of dorsal root ganglion neurons synthesizing nitric oxide synthase in different animal species.Can J Physiol Pharmacol. 2017 Apr;95(4):328-332. doi: 10.1139/cjpp-2016-0294. Epub 2016 Nov 24. Can J Physiol Pharmacol. 2017. PMID: 28103057 Review.
Cited by
-
Nitrergic modulation of ion channel function in regulating neuronal excitability.Channels (Austin). 2021 Dec;15(1):666-679. doi: 10.1080/19336950.2021.2002594. Channels (Austin). 2021. PMID: 34802368 Free PMC article.
-
Homeostatic sleep pressure is the primary factor for activation of cortical nNOS/NK1 neurons.Neuropsychopharmacology. 2015 Feb;40(3):632-9. doi: 10.1038/npp.2014.212. Epub 2014 Aug 20. Neuropsychopharmacology. 2015. PMID: 25139062 Free PMC article.
-
Investigations into Potential Extrasynaptic Communication between the Dopaminergic and Nitrergic Systems.Front Physiol. 2012 Sep 25;3:372. doi: 10.3389/fphys.2012.00372. eCollection 2012. Front Physiol. 2012. PMID: 23055978 Free PMC article.
-
NO as a multimodal transmitter in the brain: discovery and current status.Br J Pharmacol. 2019 Jan;176(2):197-211. doi: 10.1111/bph.14532. Epub 2018 Dec 5. Br J Pharmacol. 2019. PMID: 30399649 Free PMC article. Review.
-
From synaptically localized to volume transmission by nitric oxide.J Physiol. 2016 Jan 1;594(1):9-18. doi: 10.1113/JP270297. Epub 2015 Nov 18. J Physiol. 2016. PMID: 26486504 Free PMC article. Review.
References
-
- Ames W (1992) Numerical methods for partial differential equations, Ed 3. Boston: Academic.
-
- Artz JD, Toader V, Zavorin SI, Bennett BM, Thatcher GR (2001) In vitro activation of soluble guanylyl cyclase and nitric oxide release: comparison of NO donors and NO mimetics. Biochemistry 40: 9256-9264. - PubMed
-
- Bellamy TC, Griffiths C, Garthwaite J (2002) Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J Biol Chem 277: 31801-31807. - PubMed
-
- Blute TA, Lee MR, Eldred WD (2000) Direct imaging of nMDA-stimulated nitric oxide production in the retina. Vis Neurosci 17: 557-566. - PubMed
-
- Bon CL, Garthwaite J (2001) Exogenous nitric oxide causes potentiation of hippocampal synaptic transmission during low-frequency stimulation via the endogenous nitric oxide-cGMP pathway. Eur J Neurosci 14: 585-594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
