Neurocognitive and psychotiform behavioral alterations and enhanced hippocampal long-term potentiation in transgenic mice displaying neuropathological features of human alpha-mannosidosis
- PMID: 16014715
- PMCID: PMC6725435
- DOI: 10.1523/JNEUROSCI.0283-05.2005
Neurocognitive and psychotiform behavioral alterations and enhanced hippocampal long-term potentiation in transgenic mice displaying neuropathological features of human alpha-mannosidosis
Abstract
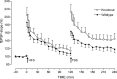
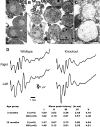
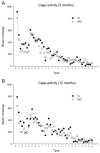
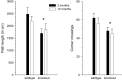
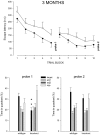
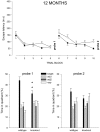
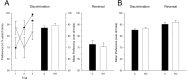
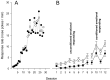
Mice with alpha-mannosidase gene inactivation provide an experimental model for alpha-mannosidosis, a lysosomal storage disease with severe neuropsychological and psychopathological complications. Neurohistological alterations in these mice were similar to those in patients and included vacuolations and axonal spheroids in the CNS and peripheral nervous system. Vacuolation was most prominent and evenly distributed in neuronal perikarya of the hippocampal CA2 and CA3 regions, whereas CA1 and dentate gyrus were weakly or not affected. Field potential recordings from CA1 region in hippocampal slices showed enhanced theta burst-induced long-term potentiation (LTP) in alpha-mannosidase-deficient mice. Longitudinal assessment in age-matched alpha-mannosidase-deficient and wild-type littermates, using an extended test battery, demonstrated a neurocognitive and psychotiform profile that may relate to the psychopathological alterations in clinical alpha-mannosidosis. Brainstem auditory-evoked potentials and basic neuromotor abilities were not impaired and did not deteriorate with age. Exploratory and conflict tests revealed consistent decreases in exploratory activity and emotional blunting in the knock-out group. alpha-Mannosidosis mice were also impaired in aversively motivated learning and acquisition of signal-shock associations. Acquisition and reversal learning in the water maze task, passive avoidance learning in the step-through procedure, as well as emotional response conditioning in an operant procedure were all impaired. Acquisition or shaping of an appetitive instrumental conditioning task was unchanged. Appetitive odor discrimination learning was only marginally impaired during shaping, whereas both the discrimination and reversal subtasks were normal. We propose that prominent storage and enhanced LTP in hippocampus have contributed to these specific behavioral alterations in alpha-mannosidase-deficient mice.
Figures

Similar articles
-
Long-term enzyme replacement therapy improves neurocognitive functioning and hippocampal synaptic plasticity in immune-tolerant alpha-mannosidosis mice.Neurobiol Dis. 2017 Oct;106:255-268. doi: 10.1016/j.nbd.2017.07.013. Epub 2017 Jul 15. Neurobiol Dis. 2017. PMID: 28720484
-
Behavioural characterisation of the alpha-mannosidosis guinea pig.Behav Brain Res. 2008 Jan 25;186(2):176-84. doi: 10.1016/j.bbr.2007.08.005. Epub 2007 Aug 10. Behav Brain Res. 2008. PMID: 17889945
-
Multivariate neurocognitive and emotional profile of a mannosidosis murine model for therapy assessment.Neurobiol Dis. 2006 Aug;23(2):422-32. doi: 10.1016/j.nbd.2006.03.009. Neurobiol Dis. 2006. PMID: 16766199
-
Lysosomal alpha-mannosidase and alpha-mannosidosis.Front Biosci (Landmark Ed). 2017 Jan 1;22(1):157-167. doi: 10.2741/4478. Front Biosci (Landmark Ed). 2017. PMID: 27814608 Review.
-
Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals.Neurobiol Learn Mem. 2015 Oct;124:3-18. doi: 10.1016/j.nlm.2015.04.006. Epub 2015 Apr 25. Neurobiol Learn Mem. 2015. PMID: 25916668 Review.
Cited by
-
Cognitive profile and activities of daily living: 35 patients with alpha-mannosidosis.J Inherit Metab Dis. 2015 Nov;38(6):1119-27. doi: 10.1007/s10545-015-9862-4. Epub 2015 May 28. J Inherit Metab Dis. 2015. PMID: 26016802
-
Nxf7 deficiency impairs social exploration and spatio-cognitive abilities as well as hippocampal synaptic plasticity in mice.Front Behav Neurosci. 2015 Jul 10;9:179. doi: 10.3389/fnbeh.2015.00179. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26217206 Free PMC article.
-
Early Cognitive and Behavioral Deficits in Mouse Models for Tauopathy and Alzheimer's Disease.Front Aging Neurosci. 2019 Dec 6;11:335. doi: 10.3389/fnagi.2019.00335. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31866856 Free PMC article.
-
Microglia lacking a peroxisomal β-oxidation enzyme chronically alter their inflammatory profile without evoking neuronal and behavioral deficits.J Neuroinflammation. 2019 Mar 13;16(1):61. doi: 10.1186/s12974-019-1442-3. J Neuroinflammation. 2019. PMID: 30866963 Free PMC article.
-
Morphological, physiological and behavioural evaluation of a 'Mice in Space' housing system.J Comp Physiol B. 2009 May;179(4):519-33. doi: 10.1007/s00360-008-0330-4. Epub 2009 Jan 8. J Comp Physiol B. 2009. PMID: 19130060 Free PMC article.
References
-
- Ara JR, Mayayo E, Marzo ME, Guelbenzu S, Chabas A, Pina MA, Calderon C (1999) Neurological impairment in α-mannosidosis: a longitudinal clinical and MRI study of a brother and sister. Childs Nerv Syst 15: 369-371. - PubMed
-
- Aronson NNJ, Kuranda MJ (1989) Lysosomal degradation of Asn-linked glycoproteins. FASEB J 3: 2615-2622. - PubMed
-
- Autio S, Louhimo T, Helenius M (1982) The clinical course of mannosidosis. Ann Clin Res 14: 93-97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
