Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury
- PMID: 16014718
- PMCID: PMC1578736
- DOI: 10.1523/JNEUROSCI.0305-05.2005
Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury
Abstract
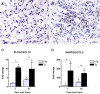
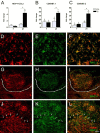
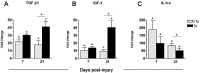
The intraspinal cues that orchestrate T-cell migration and activation after spinal contusion injury were characterized using B10.PL (wild-type) and transgenic (Tg) mice with a T-cell repertoire biased toward recognition of myelin basic protein (MBP). Previously, we showed that these strains exhibit distinct anatomical and behavioral phenotypes. In Tg mice, MBP-reactive T-cells are activated by spinal cord injury (SCI), causing more severe axonal injury, demyelination, and functional impairment than is found in non-Tg wild-type mice (B10.PL). Conversely, despite a robust SCI-induced T-cell response in B10.PL mice, no overt T-cell-mediated pathology was evident. Here, we show that chronic intraspinal T-cell accumulation in B10.PL and Tg mice is associated with a dramatic and sustained increase in CXCL10/IP-10 and CCL5/RANTES mRNA expression. However, in Tg mice, chemokine mRNA were enhanced 2- to 17-fold higher than in B10.PL mice and were associated with accelerated intraspinal T-cell influx and enhanced CNS macrophage activation throughout the spinal cord. These data suggest common molecular pathways for initiating T-cell responses after SCI in mice; however, if T-cell reactions are biased against MBP, molecular and cellular determinants of neuroinflammation are magnified in parallel with exacerbation of neuropathology and functional impairment.
Figures

Similar articles
-
Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat.J Neurosci Res. 2001 Jun 15;64(6):582-9. doi: 10.1002/jnr.1110. J Neurosci Res. 2001. PMID: 11398181
-
Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury.J Neurosci Res. 1998 Aug 1;53(3):368-76. doi: 10.1002/(SICI)1097-4547(19980801)53:3<368::AID-JNR11>3.0.CO;2-1. J Neurosci Res. 1998. PMID: 9698165
-
Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy.J Neurosci. 2002 Apr 1;22(7):2690-700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. J Neurosci. 2002. PMID: 11923434 Free PMC article.
-
Autoimmunity can benefit self-maintenance.Immunol Today. 2000 Jun;21(6):265-8. doi: 10.1016/s0167-5699(00)01633-9. Immunol Today. 2000. PMID: 10825737 Review.
-
Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury.Neuroscience. 2009 Feb 6;158(3):1112-21. doi: 10.1016/j.neuroscience.2008.07.001. Epub 2008 Jul 4. Neuroscience. 2009. PMID: 18674593 Free PMC article. Review.
Cited by
-
Potential immunotherapies for traumatic brain and spinal cord injury.Chin J Traumatol. 2018 Jun;21(3):125-136. doi: 10.1016/j.cjtee.2018.02.002. Epub 2018 Apr 18. Chin J Traumatol. 2018. PMID: 29759918 Free PMC article. Review.
-
The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury.Exp Neurol. 2014 Aug;258:121-129. doi: 10.1016/j.expneurol.2014.04.023. Exp Neurol. 2014. PMID: 25017893 Free PMC article. Review.
-
Chemokines as possible targets in modulation of the secondary damage after acute spinal cord injury: a review.Cell Mol Neurobiol. 2009 Sep;29(6-7):1025-35. doi: 10.1007/s10571-009-9392-4. Epub 2009 Apr 11. Cell Mol Neurobiol. 2009. PMID: 19363652 Free PMC article. Review.
-
Syndromics: a bioinformatics approach for neurotrauma research.Transl Stroke Res. 2011 Dec;2(4):438-54. doi: 10.1007/s12975-011-0121-1. Epub 2011 Nov 18. Transl Stroke Res. 2011. PMID: 22207883 Free PMC article.
-
Role of secretory phospholipase a(2) in CNS inflammation: implications in traumatic spinal cord injury.CNS Neurol Disord Drug Targets. 2008 Jun;7(3):254-69. doi: 10.2174/187152708784936671. CNS Neurol Disord Drug Targets. 2008. PMID: 18673210 Free PMC article. Review.
References
-
- Appay V, Dunbar PR, Cerundolo V, McMichael A, Czaplewski L, Rowland-Jones S (2000) RANTES activates antigen-specific cytotoxic T lymphocytes in a mitogen-like manner through cell surface aggregation. Int Immunol 12: 1173-1182. - PubMed
-
- Bacon KB, Premack BA, Gardner P, Schall TJ (1995) Activation of dual T cell signaling pathways by the chemokine RANTES. Science 269: 1727-1730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
