Rat adrenal chromaffin cells are neonatal CO2 sensors
- PMID: 16014724
- PMCID: PMC6725439
- DOI: 10.1523/JNEUROSCI.1139-05.2005
Rat adrenal chromaffin cells are neonatal CO2 sensors
Abstract
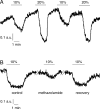
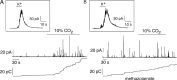
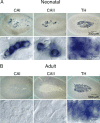
We studied the participation of adrenal medulla (AM) chromaffin cells in hypercapnic chemotransduction. Using amperometric recordings, we measured catecholamine (CAT) secretion from cells in AM slices of neonatal and adult rats perfused with solutions bubbled with different concentrations of CO2. The secretory activity augmented from 1.74 +/- 0.19 pC/min at 5% CO2 to 6.36 +/- 0.77 pC/min at 10% CO2. This response to CO2 was dose dependent and appeared without changes in extracellular pH, although it was paralleled by a drop in intracellular pH. Responsiveness to hypercapnia was higher in neonatal than in adult slices. The secretory response to hypercapnia required extracellular Ca2+ influx. Both the CO2-induced internal pH drop and increase in CAT secretion were markedly diminished by methazolamide (2 microm), a membrane-permeant carbonic anhydrase (CA) inhibitor. We detected the presence of two CA isoforms (CAI and CAII) in neonatal AM slices by in situ hybridization and real-time PCR. The expression of these enzymes decreased in adult AM together with the disappearance of responsiveness to CO2. In patch-clamped chromaffin cells, hypercapnia elicited a depolarizing receptor potential, which led to action potential firing, extracellular Ca2+ influx, and CAT secretion. This receptor potential (inhibited by methazolamide) was primarily attributable to activation of a resting cationic conductance. In addition, voltage-gated K+ current amplitude was also decreased by high CO2. The CO2-sensing properties of chromaffin cells may be of physiologic relevance, particularly for the adaptation of neonates to extrauterine life, before complete maturation of peripheral and central chemoreceptors.
Figures





Similar articles
-
Chronic opioids regulate KATP channel subunit Kir6.2 and carbonic anhydrase I and II expression in rat adrenal chromaffin cells via HIF-2α and protein kinase A.Am J Physiol Cell Physiol. 2014 Aug 1;307(3):C266-77. doi: 10.1152/ajpcell.00135.2014. Epub 2014 Jun 4. Am J Physiol Cell Physiol. 2014. PMID: 24898587 Free PMC article.
-
Inhibition of voltage-gated Ca(2+) current by PACAP in rat adrenal chromaffin cells.Regul Pept. 2002 Jan 15;103(1):59-65. doi: 10.1016/s0167-0115(01)00327-5. Regul Pept. 2002. PMID: 11738249
-
Functional mitochondria are required for O2 but not CO2 sensing in immortalized adrenomedullary chromaffin cells.Am J Physiol Cell Physiol. 2008 Apr;294(4):C945-56. doi: 10.1152/ajpcell.00495.2007. Epub 2008 Jan 30. Am J Physiol Cell Physiol. 2008. PMID: 18234847
-
Carotid body chemoreception: the importance of CO2-HCO3- and carbonic anhydrase. (review).Biol Res. 1993;26(3):319-29. Biol Res. 1993. PMID: 7606251 Review.
-
Ontogeny of O2 and CO2//H+ chemosensitivity in adrenal chromaffin cells: role of innervation.J Exp Biol. 2014 Mar 1;217(Pt 5):673-81. doi: 10.1242/jeb.086165. J Exp Biol. 2014. PMID: 24574383 Review.
Cited by
-
Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress.J Physiol. 2006 Aug 15;575(Pt 1):229-39. doi: 10.1113/jphysiol.2006.112524. Epub 2006 Jun 15. J Physiol. 2006. PMID: 16777938 Free PMC article.
-
Effect of carbon dioxide on calcitonin gene-related peptide secretion from trigeminal neurons.Headache. 2007 Nov-Dec;47(10):1385-97. doi: 10.1111/j.1526-4610.2007.00850.x. Headache. 2007. PMID: 18052948 Free PMC article.
-
The locus coeruleus and central chemosensitivity.Respir Physiol Neurobiol. 2010 Oct 31;173(3):264-73. doi: 10.1016/j.resp.2010.04.024. Epub 2010 May 8. Respir Physiol Neurobiol. 2010. PMID: 20435170 Free PMC article. Review.
-
Chronic opioids regulate KATP channel subunit Kir6.2 and carbonic anhydrase I and II expression in rat adrenal chromaffin cells via HIF-2α and protein kinase A.Am J Physiol Cell Physiol. 2014 Aug 1;307(3):C266-77. doi: 10.1152/ajpcell.00135.2014. Epub 2014 Jun 4. Am J Physiol Cell Physiol. 2014. PMID: 24898587 Free PMC article.
-
Gene expression analyses reveal metabolic specifications in acute O2 -sensing chemoreceptor cells.J Physiol. 2017 Sep 15;595(18):6091-6120. doi: 10.1113/JP274684. Epub 2017 Aug 8. J Physiol. 2017. PMID: 28718507 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
