Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain
- PMID: 16014727
- PMCID: PMC6725431
- DOI: 10.1523/JNEUROSCI.1490-05.2005
Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain
Abstract
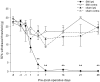
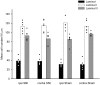
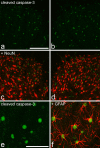
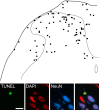
It has been proposed that death of inhibitory interneurons in the dorsal horn contributes to the neuropathic pain that follows partial nerve injury. In this study, we have used two approaches to test whether there is neuronal death in the dorsal horn in the spared nerve injury (SNI) model. We performed a stereological analysis of the packing density of neurons in laminas I-III 4 weeks after operation and found no reduction on the ipsilateral side compared with that seen on the contralateral side or in sham-operated or naive rats. In addition, we used two markers of apoptosis, terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining and immunocytochemical detection of cleaved (activated) caspase-3. Neither of these methods demonstrated apoptotic neurons in the dorsal spinal cord 1 week after operation. Although TUNEL-positive cells were present throughout the gray and white matter at this stage, they were virtually all labeled with antibody against ionized calcium-binding adapter molecule 1, a marker for microglia. All animals that underwent SNI showed clear signs of tactile allodynia affecting the ipsilateral hindpaw. These results suggest that a significant loss of neurons from the dorsal horn is not necessary for the development of tactile allodynia in the SNI model.
Figures


Similar articles
-
Tactile allodynia can occur in the spared nerve injury model in the rat without selective loss of GABA or GABA(A) receptors from synapses in laminae I-II of the ipsilateral spinal dorsal horn.Neuroscience. 2008 Sep 22;156(1):193-202. doi: 10.1016/j.neuroscience.2008.07.009. Epub 2008 Jul 10. Neuroscience. 2008. PMID: 18675320 Free PMC article.
-
Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord.J Neurosci. 2002 Aug 1;22(15):6724-31. doi: 10.1523/JNEUROSCI.22-15-06724.2002. J Neurosci. 2002. PMID: 12151551 Free PMC article.
-
Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections.Pain. 2001 Feb 1;90(1-2):105-11. doi: 10.1016/s0304-3959(00)00392-4. Pain. 2001. PMID: 11166976
-
Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury.J Neurosci. 2005 Aug 10;25(32):7317-23. doi: 10.1523/JNEUROSCI.1526-05.2005. J Neurosci. 2005. PMID: 16093381 Free PMC article.
-
Peripheral afferents and spinal inhibitory system in dynamic and static mechanical allodynia.Pain. 2017 Dec;158(12):2285-2289. doi: 10.1097/j.pain.0000000000001055. Pain. 2017. PMID: 28885453 Free PMC article. Review. No abstract available.
Cited by
-
Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn.Pain. 2013 Dec;154(12):2606-2615. doi: 10.1016/j.pain.2013.05.001. Epub 2013 May 7. Pain. 2013. PMID: 23707280 Free PMC article.
-
Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: an electrophysiological and immunohistochemical study.J Neurosci. 2009 Oct 7;29(40):12532-41. doi: 10.1523/JNEUROSCI.2887-09.2009. J Neurosci. 2009. PMID: 19812328 Free PMC article.
-
Autophagy impairment in a mouse model of neuropathic pain.Mol Pain. 2011 Oct 24;7:83. doi: 10.1186/1744-8069-7-83. Mol Pain. 2011. PMID: 22023914 Free PMC article.
-
Protein Kinase A Is Involved in Neuropathic Pain by Activating the p38MAPK Pathway to Mediate Spinal Cord Cell Apoptosis.Mediators Inflamm. 2020 Mar 23;2020:6420425. doi: 10.1155/2020/6420425. eCollection 2020. Mediators Inflamm. 2020. PMID: 32273830 Free PMC article.
-
Spatial and temporal pattern of changes in the number of GAD65-immunoreactive inhibitory terminals in the rat superficial dorsal horn following peripheral nerve injury.Mol Pain. 2014 Sep 4;10:57. doi: 10.1186/1744-8069-10-57. Mol Pain. 2014. PMID: 25189404 Free PMC article.
References
-
- Araki T, Simon RP, Taki W, Lan JQ, Henshall DC (2002) Characterization of neuronal death induced by focally evoked limbic seizures in the C57BL/6 mouse. J Neurosci Res 69: 614-621. - PubMed
-
- Azkue JJ, Zimmermann M, Hsieh T-F, Herdegen T (1998) Peripheral nerve insult induces NMDA receptor-mediated, delayed degeneration in spinal neurons. Eur J Neurosci 10: 2204-2206. - PubMed
-
- Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33: 87-107. - PubMed
-
- Bjugn R, Gundersen HJ (1993) Estimate of the total number of neurons and glial and endothelial cells in the rat spinal cord by means of the optical disector. J Comp Neurol 328: 406-414. - PubMed
-
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE (1988) Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 32: 89-94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
