Identification of secret agent as the O-GlcNAc transferase that participates in Plum pox virus infection
- PMID: 16014901
- PMCID: PMC1181581
- DOI: 10.1128/JVI.79.15.9381-9387.2005
Identification of secret agent as the O-GlcNAc transferase that participates in Plum pox virus infection
Abstract
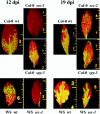
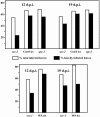
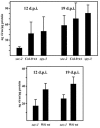
Serine and threonine of many nuclear and cytoplasmic proteins are posttranslationally modified with O-linked N-acetylglucosamine (O-GlcNAc). This modification is made by O-linked N-acetylglucosamine transferases (OGTs). Genetic and biochemical data have demonstrated the existence of two OGTs of Arabidopsis thaliana, SECRET AGENT (SEC) and SPINDLY (SPY), with at least partly overlapping functions, but there is little information on their target proteins. The N terminus of the capsid protein (CP) of Plum pox virus (PPV) isolated from Nicotiana clevelandii is O-GlcNAc modified. We show here that O-GlcNAc modification of PPV CP also takes place in other plant hosts, N. benthamiana and Arabidopsis. PPV was able to infect the Arabidopsis OGT mutants sec-1, sec-2, and spy-3, but at early times of the infection, both rate of virus spread and accumulation were reduced in sec-1 and sec-2 relative to spy-3 and wild-type plants. By matrix-assisted laser desorption ionization-time of flight mass spectrometry, we determined that a 39-residue tryptic peptide from the N terminus of CP of PPV purified from the spy-3 mutant, but not sec-1 or sec-2, was O-GlcNAc modified, suggesting that SEC but not SPY modifies the capsid. While our results indicate that O-GlcNAc modification of PPV CP by SEC is not essential for infection, they show that the modification has a role(s) in the process.
Figures

Similar articles
-
O-GlcNAcylation of the Plum pox virus capsid protein catalyzed by SECRET AGENT: characterization of O-GlcNAc sites by electron transfer dissociation mass spectrometry.Amino Acids. 2011 Mar;40(3):869-76. doi: 10.1007/s00726-010-0706-0. Epub 2010 Jul 31. Amino Acids. 2011. PMID: 20676902 Free PMC article.
-
O-GlcNAc modification of the coat protein of the potyvirus Plum pox virus enhances viral infection.Virology. 2013 Aug 1;442(2):122-31. doi: 10.1016/j.virol.2013.03.029. Epub 2013 Apr 29. Virology. 2013. PMID: 23639873 Free PMC article.
-
SECRET AGENT, an Arabidopsis thaliana O-GlcNAc transferase, modifies the Plum pox virus capsid protein.FEBS Lett. 2006 Oct 30;580(25):5829-35. doi: 10.1016/j.febslet.2006.09.046. Epub 2006 Sep 29. FEBS Lett. 2006. PMID: 17027982
-
O-GlcNAc protein modification in plants: Evolution and function.Biochim Biophys Acta. 2010 Feb;1800(2):49-56. doi: 10.1016/j.bbagen.2009.11.016. Epub 2009 Dec 2. Biochim Biophys Acta. 2010. PMID: 19961900 Free PMC article. Review.
-
Novel nucleocytoplasmic protein O-fucosylation by SPINDLY regulates diverse developmental processes in plants.Curr Opin Struct Biol. 2021 Jun;68:113-121. doi: 10.1016/j.sbi.2020.12.013. Epub 2021 Jan 18. Curr Opin Struct Biol. 2021. PMID: 33476897 Free PMC article. Review.
Cited by
-
Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer.Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):11952-7. doi: 10.1073/pnas.0601931103. Epub 2006 Aug 1. Proc Natl Acad Sci U S A. 2006. PMID: 16882729 Free PMC article.
-
A novel role of the potyviral helper component proteinase contributes to enhance the yield of viral particles.J Virol. 2014 Sep 1;88(17):9808-18. doi: 10.1128/JVI.01010-14. Epub 2014 Jun 18. J Virol. 2014. PMID: 24942578 Free PMC article.
-
Altered gene expression changes in Arabidopsis leaf tissues and protoplasts in response to Plum pox virus infection.BMC Genomics. 2008 Jul 9;9:325. doi: 10.1186/1471-2164-9-325. BMC Genomics. 2008. PMID: 18613973 Free PMC article.
-
O-GlcNAcylation of the Plum pox virus capsid protein catalyzed by SECRET AGENT: characterization of O-GlcNAc sites by electron transfer dissociation mass spectrometry.Amino Acids. 2011 Mar;40(3):869-76. doi: 10.1007/s00726-010-0706-0. Epub 2010 Jul 31. Amino Acids. 2011. PMID: 20676902 Free PMC article.
-
O-GlcNAc modification of the coat protein of the potyvirus Plum pox virus enhances viral infection.Virology. 2013 Aug 1;442(2):122-31. doi: 10.1016/j.virol.2013.03.029. Epub 2013 Apr 29. Virology. 2013. PMID: 23639873 Free PMC article.
References
-
- Baratova, L. A., N. V. Fedorova, E. N. Dobrov, E. V. Lukashina, A. N. Kharlanov, V. V. Nasonov, M. V. Serebryakova, S. V. Kozlovsky, O. V. Zayakina, and N. P. Rodionova. 2004. N-terminal segment of potato virus X coat protein subunits is glycosylated and mediates formation of a bound water shell on the virion surface. Eur. J. Biochem. 271:3136-3145. - PubMed
-
- Comer, F. I., and G. W. Hart. 2000. O-glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J. Biol. Chem. 275:29179-29182. - PubMed
-
- Fernández-Fernández, M. R., E. Camafeita, P. Bonay, E. Méndez, J. P. Albar, and J. A. García. 2002. The capsid protein of a plant single-stranded RNA virus is modified by O-linked N-acetylglucosamine. J. Biol. Chem. 277:135-140. - PubMed
-
- Fernández-Fernández, M. R., J. L. Martínez-Torrecuadrada, J. I. Casal, and J. A. García. 1998. Development of an antigen presentation system based on plum pox potyvirus. FEBS Lett. 427:229-235. - PubMed
-
- Fernández-Fernández, M. R., M. Mouriño, J. Rivera, F. Rodríguez, J. Plana-Durán, and J. A. García. 2001. Protection of rabbits against rabbit hemorrhagic disease virus by immunization with the VP60 protein expressed in plants with a potyvirus-based vector. Virology 280:283-291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

