Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T-cell receptor beta-deficient mice infected with a coronavirus
- PMID: 16014902
- PMCID: PMC1181615
- DOI: 10.1128/JVI.79.15.9388-9396.2005
Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T-cell receptor beta-deficient mice infected with a coronavirus
Abstract
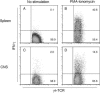
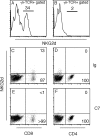
gammadelta T cells mediate demyelination in athymic (nude) mice infected with the neurotropic coronavirus mouse hepatitis virus strain JHM. Now, we show that these cells also mediate the same process in mice lacking alphabeta T cells (T-cell receptor beta-deficient [TCRbeta(-/-)] mice) and demyelination is gamma interferon (IFN-gamma) dependent. Most strikingly, our results also show a major role for NKG2D, expressed on gammadelta T cells, in the demyelinating process with in vivo blockade of NKG2D interactions resulting in a 60% reduction in demyelination. NKG2D may serve as a primary recognition receptor or as a costimulatory molecule. We show that NKG2D(+) gammadelta T cells in the JHM-infected central nervous system express the adaptor molecule DAP12 and an NKG2D isoform (NKG2D short), both required for NKG2D to serve as a primary receptor. These results are consistent with models in which gammadelta T cells mediate demyelination using the same effector cytokine, IFN-gamma, as CD8 T cells and do so without a requirement for signaling through the TCR.
Figures
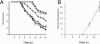



References
-
- Backstrom, E., B. J. Chambers, E. L. Ho, O. V. Naidenko, R. Mariotti, D. H. Fremont, W. M. Yokoyama, K. Kristensson, and H. G. Ljunggren. 2003. Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions. Eur. J. Immunol. 33:92-100. - PubMed
-
- Bauer, S., V. Groh, J. Wu, A. Steinle, J. H. Phillips, L. L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727-729. - PubMed
-
- Benveniste, P., B. S. Chadwick, R. G. Miller, and J. Reimann. 1990. Characterization of cells with T-cell markers in athymic nude bone marrow and of their in vitro-derived clonal progeny. Comparison with euthymic bone marrow. J. Immunol. 144:411-419. - PubMed
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

