Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection
- PMID: 16014905
- PMCID: PMC1181607
- DOI: 10.1128/JVI.79.15.9419-9429.2005
Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection
Abstract
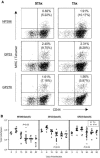
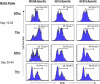
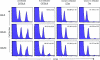
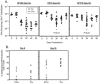
Although it is well documented that CD8 T cells play a critical role in controlling chronic viral infections, the mechanisms underlying the regulation of CD8 T-cell responses are not well understood. Using the mouse model of an acute and chronic lymphocytic choriomeningitis virus (LCMV) infection, we have examined the relative importance of peripheral T cells and thymic emigrants in the elicitation and maintenance of CD8 T-cell responses. Virus-specific CD8 T-cell responses were compared between mice that were either sham thymectomized or thymectomized (Thx) at approximately 6 weeks of age. In an acute LCMV infection, thymic deficiency did not affect either the primary expansion of CD8 T cells or the proliferative renewal and maintenance of virus-specific lymphoid and nonlymphoid memory CD8 T cells. Following a chronic LCMV infection, in Thx mice, although the initial expansion of CD8 T cells was normal, the contraction phase of the CD8 T-cell response was exaggerated, which led to a transient but striking CD8 T-cell deficit on day 30 postinfection. However, the virus-specific CD8 T-cell response in Thx mice rebounded quickly and was maintained at normal levels thereafter, which indicated that the peripheral T-cell repertoire is quite robust and capable of sustaining an effective CD8 T-cell response in the absence of thymic output during a chronic LCMV infection. Taken together, these findings should further our understanding of the regulation of CD8 T-cell homeostasis in acute and chronic viral infections and might have implications in the development of immunotherapy.
Figures





Similar articles
-
Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection.J Virol. 2005 Jan;79(1):202-13. doi: 10.1128/JVI.79.1.202-213.2005. J Virol. 2005. PMID: 15596816 Free PMC article.
-
Effect of chronic viral infection on epitope selection, cytokine production, and surface phenotype of CD8 T cells and the role of IFN-gamma receptor in immune regulation.J Immunol. 2004 Feb 1;172(3):1491-500. doi: 10.4049/jimmunol.172.3.1491. J Immunol. 2004. PMID: 14734726
-
PD-L1 Checkpoint Inhibition Narrows the Antigen-Specific T Cell Receptor Repertoire in Chronic Lymphocytic Choriomeningitis Virus Infection.J Virol. 2020 Aug 31;94(18):e00795-20. doi: 10.1128/JVI.00795-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641478 Free PMC article.
-
T cell responses to viral infections: lessons from lymphocytic choriomeningitis virus.Immunol Res. 2002;26(1-3):309-21. doi: 10.1385/IR:26:1-3:309. Immunol Res. 2002. PMID: 12403369 Review.
-
Complexities of Type I Interferon Biology: Lessons from LCMV.Viruses. 2019 Feb 20;11(2):172. doi: 10.3390/v11020172. Viruses. 2019. PMID: 30791575 Free PMC article. Review.
Cited by
-
Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection.J Exp Med. 2006 Oct 2;203(10):2263-9. doi: 10.1084/jem.20060995. Epub 2006 Sep 11. J Exp Med. 2006. PMID: 16966427 Free PMC article.
-
When the Damage Is Done: Injury and Repair in Thymus Function.Front Immunol. 2020 Aug 12;11:1745. doi: 10.3389/fimmu.2020.01745. eCollection 2020. Front Immunol. 2020. PMID: 32903477 Free PMC article. Review.
-
Molecular and cellular insights into T cell exhaustion.Nat Rev Immunol. 2015 Aug;15(8):486-99. doi: 10.1038/nri3862. Nat Rev Immunol. 2015. PMID: 26205583 Free PMC article. Review.
-
Infection of adult thymus with murine retrovirus induces virus-specific central tolerance that prevents functional memory CD8+ T cell differentiation.PLoS Pathog. 2014 Mar 20;10(3):e1003937. doi: 10.1371/journal.ppat.1003937. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24651250 Free PMC article.
-
Post-thymic maturation: young T cells assert their individuality.Nat Rev Immunol. 2011 Jul 22;11(8):544-9. doi: 10.1038/nri3028. Nat Rev Immunol. 2011. PMID: 21779032 Free PMC article. Review.
References
-
- Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. - PMC - PubMed
-
- Appay, V., L. Papagno, C. A. Spina, P. Hansasuta, A. King, L. Jones, G. S. Ogg, S. Little, A. J. McMichael, D. D. Richman, and S. L. Rowland-Jones. 2002. Dynamics of T cell responses in HIV infection. J. Immunol. 168:3660-3666. - PubMed
-
- Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 3:619-626. - PubMed
-
- Barry, S. M., M. A. Johnson, and G. Janossy. 2003. Increased proportions of activated and proliferating memory CD8+ T lymphocytes in both blood and lung are associated with blood HIV viral load. J. Acquir. Immune Defic. Syndr. 34:351-357. - PubMed
-
- Benlhassan-Chahour, K., C. Penit, V. Dioszeghy, F. Vasseur, G. Janvier, Y. Riviere, N. Dereuddre-Bosquet, D. Dormont, R. Le Grand, and B. Vaslin. 2003. Kinetics of lymphocyte proliferation during primary immune response in macaques infected with pathogenic simian immunodeficiency virus SIVmac251: preliminary report of the effect of early antiviral therapy. J. Virol. 77:12479-12493. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

