Vaccinia virus A21 virion membrane protein is required for cell entry and fusion
- PMID: 16014909
- PMCID: PMC1181583
- DOI: 10.1128/JVI.79.15.9458-9469.2005
Vaccinia virus A21 virion membrane protein is required for cell entry and fusion
Abstract
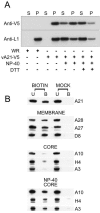
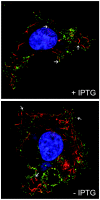
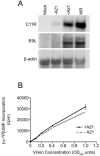
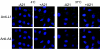
We provide the initial characterization of the product of the vaccinia virus A21L (VACWR140) gene and demonstrate that it is required for cell entry and low pH-triggered membrane fusion. The A21L open reading frame, which is conserved in all sequenced members of the poxvirus family, encodes a protein of 117 amino acids with an N-terminal hydrophobic domain and four invariant cysteines. Expression of the A21 protein occurred at late times of infection and was dependent on viral DNA replication. The A21 protein contained two intramolecular disulfide bonds, the formation of which required the vaccinia virus-encoded cytoplasmic redox pathway, and was localized on the surface of the lipoprotein membrane of intracellular mature virions. A conditional lethal mutant, in which A21L gene expression was regulated by isopropyl-beta-d-thiogalactopyranoside, was constructed. In the absence of inducer, cell-to-cell spread of virus did not occur, despite the formation of morphologically normal intracellular virions and extracellular virions with actin tails. Purified virions lacking A21 were able to bind to cells, but cores did not penetrate into the cytoplasm and synthesize viral RNA. In addition, virions lacking A21 were unable to mediate low pH-triggered cell-cell fusion. The A21 protein, like the A28 and H2 proteins, is an essential component of the poxvirus entry/fusion apparatus for both intracellular and extracellular virus particles.
Figures




References
-
- Ahn, B.-Y., P. D. Gershon, and B. Moss. 1994. RNA-polymerase associated protein RAP94 confers promoter specificity for initiating transcription of vaccinia virus early stage genes. J. Biol. Chem. 269:7552-7557. - PubMed
-
- Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. - PubMed
-
- Bablanian, R., B. Baxt, J. A. Sonnabend, and M. Esteban. 1978. Studies on the mechanisms of vaccinia virus cytopathic effects. II. Early cell rounding is associated with virus polypeptide synthesis. J. Gen. Virol. 39:403-413. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

