Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells
- PMID: 16014923
- PMCID: PMC1181545
- DOI: 10.1128/JVI.79.15.9608-9617.2005
Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells
Abstract
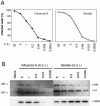
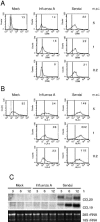
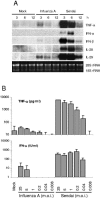
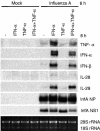
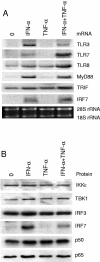
Dendritic cells (DCs) respond to microbial infections by undergoing phenotypic maturation and by producing multiple cytokines. In the present study, we analyzed the ability of influenza A and Sendai viruses to induce DC maturation and activate tumor necrosis factor alpha (TNF-alpha), alpha/beta interferon (IFN-alpha/beta), and IFN-like interleukin-28A/B (IFN-lambda2/3) and IL-29 (IFN-lambda1) gene expression in human monocyte-derived myeloid DCs (mDC). The ability of influenza A virus to induce mDC maturation or enhance the expression of TNF-alpha, IFN-alpha/beta, interleukin-28 (IL-28), and IL-29 genes was limited, whereas Sendai virus efficiently induced mDC maturation and enhanced cytokine gene expression. Influenza A virus-induced expression of TNF-alpha, IFN-alpha, IFN-beta, IL-28, and IL-29 genes was, however, dramatically enhanced when cells were pretreated with IFN-alpha. IFN-alpha priming led to increased expression of Toll-like receptor 3 (TLR3), TLR7, TLR8, MyD88, TRIF, and IFN regulatory factor 7 (IRF7) genes and enhanced influenza-induced phosphorylation and DNA binding of IRF3. Influenza A virus also enhanced the binding of NF-kappaB to the respective NF-kappaB elements of the promoters of IFN-beta and IL-29 genes. In mDC IL-29 induced MxA protein expression and possessed antiviral activity against influenza A virus, although this activity was lower than that of IFN-alpha or IFN-beta. Our results show that in human mDCs viruses can readily induce the expression of IL-28 and IL-29 genes whose gene products are likely to contribute to the host antiviral response.
Figures

Similar articles
-
IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29.J Immunol. 2005 Feb 15;174(4):1932-7. doi: 10.4049/jimmunol.174.4.1932. J Immunol. 2005. PMID: 15699120
-
Toll-like receptor 3 mediates a more potent antiviral response than Toll-like receptor 4.J Immunol. 2003 Apr 1;170(7):3565-71. doi: 10.4049/jimmunol.170.7.3565. J Immunol. 2003. PMID: 12646618
-
Early alpha/beta interferon production by myeloid dendritic cells in response to UV-inactivated virus requires viral entry and interferon regulatory factor 3 but not MyD88.J Virol. 2005 Aug;79(16):10376-85. doi: 10.1128/JVI.79.16.10376-10385.2005. J Virol. 2005. PMID: 16051830 Free PMC article.
-
[Toll-like receptors that sense viral infection].Uirusu. 2004 Jun;54(1):1-8. doi: 10.2222/jsv.54.1. Uirusu. 2004. PMID: 15449898 Review. Japanese.
-
Toll-like receptor 3: a link between toll-like receptor, interferon and viruses.Microbiol Immunol. 2004;48(3):147-54. doi: 10.1111/j.1348-0421.2004.tb03500.x. Microbiol Immunol. 2004. PMID: 15031527 Review.
Cited by
-
Sendai virus, an RNA virus with no risk of genomic integration, delivers CRISPR/Cas9 for efficient gene editing.Mol Ther Methods Clin Dev. 2016 Aug 24;3:16057. doi: 10.1038/mtm.2016.57. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27606350 Free PMC article.
-
Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo.J Virol. 2010 Nov;84(21):11515-22. doi: 10.1128/JVI.01703-09. Epub 2010 Aug 25. J Virol. 2010. PMID: 20739515 Free PMC article.
-
Highly Pathogenic H5N1 Influenza A Virus Spreads Efficiently in Human Primary Monocyte-Derived Macrophages and Dendritic Cells.Front Immunol. 2018 Jul 17;9:1664. doi: 10.3389/fimmu.2018.01664. eCollection 2018. Front Immunol. 2018. PMID: 30065728 Free PMC article.
-
IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo.PLoS Pathog. 2008 Mar 14;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. PLoS Pathog. 2008. PMID: 18369468 Free PMC article.
-
Novel Types of Small RNA Exhibit Sequence- and Target-dependent Angiogenesis Suppression Without Activation of Toll-like Receptor 3 in an Age-related Macular Degeneration (AMD) Mouse Model.Mol Ther Nucleic Acids. 2015 Oct 20;4(10):e258. doi: 10.1038/mtna.2015.34. Mol Ther Nucleic Acids. 2015. PMID: 26484944 Free PMC article.
References
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. - PubMed
-
- Au, W. C., W. S. Yeow, and P. M. Pitha. 2001. Analysis of functional domains of interferon regulatory factor 7 and its association with IRF-3. Virology 280:273-282. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Barchet, W., A. Krug, M. Cella, C. Newby, J. A. Fischer, A. Dzionek, A. Pekosz, and M. Colonna. 2005. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. 35:236-242. - PubMed
-
- Benvenuti, F., C. Lagaudriere-Gesbert, I. Grandjean, C. Jancic, C. Hivroz, A. Trautmann, O. Lantz, and S. Amigorena. 2004. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J. Immunol. 172:292-301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

