Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity
- PMID: 16014931
- PMCID: PMC1181575
- DOI: 10.1128/JVI.79.15.9694-9701.2005
Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity
Abstract
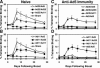
The high prevalence of preexisting immunity to adenovirus serotype 5 (Ad5) in human populations will likely limit the immunogenicity and clinical utility of recombinant Ad5 (rAd5) vector-based vaccines for human immunodeficiency virus type 1 and other pathogens. A potential solution to this problem is to utilize rAd vaccine vectors derived from rare Ad serotypes such as Ad35 and Ad11. We have previously reported that rAd35 vectors were immunogenic in the presence of anti-Ad5 immunity, but the immunogenicity of heterologous rAd prime-boost regimens and the extent that cross-reactive anti-vector immunity may limit this approach have not been fully explored. Here we assess the immunogenicity of heterologous vaccine regimens involving rAd5, rAd35, and novel rAd11 vectors expressing simian immunodeficiency virus Gag in mice both with and without anti-Ad5 immunity. Heterologous rAd prime-boost regimens proved significantly more immunogenic than homologous regimens, as expected. Importantly, all regimens that included rAd5 were markedly suppressed by anti-Ad5 immunity. In contrast, rAd35-rAd11 and rAd11-rAd35 regimens elicited high-frequency immune responses both in the presence and in the absence of anti-Ad5 immunity, although we also detected clear cross-reactive Ad35/Ad11-specific humoral and cellular immune responses. Nevertheless, these data suggest the potential utility of heterologous rAd prime-boost vaccine regimens using vectors derived from rare human Ad serotypes.
Figures

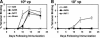
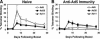

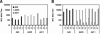

Similar articles
-
Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity.J Virol. 2006 Dec;80(24):12009-16. doi: 10.1128/JVI.01749-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035318 Free PMC article.
-
Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity.J Immunol. 2004 May 15;172(10):6290-7. doi: 10.4049/jimmunol.172.10.6290. J Immunol. 2004. PMID: 15128818
-
Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors.J Gen Virol. 2006 Jan;87(Pt 1):139-149. doi: 10.1099/vir.0.81445-0. J Gen Virol. 2006. PMID: 16361426
-
Novel adenovirus vector-based vaccines for HIV-1.Curr Opin HIV AIDS. 2010 Sep;5(5):386-90. doi: 10.1097/COH.0b013e32833cfe4c. Curr Opin HIV AIDS. 2010. PMID: 20978378 Free PMC article. Review.
-
Development and evaluation of a novel gene delivery vehicle composed of adenovirus serotype 35.Biol Pharm Bull. 2008 Oct;31(10):1819-25. doi: 10.1248/bpb.31.1819. Biol Pharm Bull. 2008. PMID: 18827334 Review.
Cited by
-
Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone.Infect Immun. 2007 May;75(5):2283-90. doi: 10.1128/IAI.01879-06. Epub 2007 Feb 16. Infect Immun. 2007. PMID: 17307942 Free PMC article.
-
mRNA-Based Vaccines and Mode of Action.Curr Top Microbiol Immunol. 2022;440:1-30. doi: 10.1007/82_2020_230. Curr Top Microbiol Immunol. 2022. PMID: 33591423 Review.
-
Control of SIV infection and subsequent induction of pandemic H1N1 immunity in rhesus macaques using an Ad5 [E1-, E2b-] vector platform.Vaccine. 2012 Nov 26;30(50):7265-70. doi: 10.1016/j.vaccine.2012.09.058. Epub 2012 Oct 2. Vaccine. 2012. PMID: 23041546 Free PMC article.
-
Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies.J Virol. 2006 Dec;80(24):11991-7. doi: 10.1128/JVI.01348-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005652 Free PMC article.
-
Hantavirus entry: Perspectives and recent advances.Adv Virus Res. 2019;104:185-224. doi: 10.1016/bs.aivir.2019.07.002. Epub 2019 Aug 7. Adv Virus Res. 2019. PMID: 31439149 Free PMC article.
References
-
- Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting HIV-1 vaccines. J. Virol. 77:8729-8735. - PMC - PubMed
-
- Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of preexisting anti-Ad5 immunity. J. Immunol. 172:6290-6297. - PubMed
-
- Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

