Persistence of bovine viral diarrhea virus is determined by a cellular cofactor of a viral autoprotease
- PMID: 16014936
- PMCID: PMC1181585
- DOI: 10.1128/JVI.79.15.9746-9755.2005
Persistence of bovine viral diarrhea virus is determined by a cellular cofactor of a viral autoprotease
Abstract
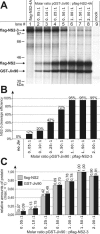
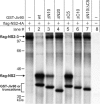
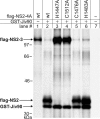
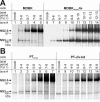
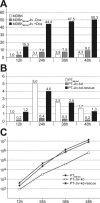
Polyprotein processing control is a crucial step in the life cycle of positive-strand RNA viruses. Recently, a vital autoprotease generating an essential viral replication factor was identified in such a virus, namely, the pestivirus bovine viral diarrhea virus. Surprisingly, the activity of this protease, which resides in nonstructural protein 2 (NS2), diminishes early after infection, resulting in the limitation of viral RNA replication. Here, we describe that a cellular chaperone termed Jiv (J-domain protein interacting with viral protein) acts as a cofactor of the NS2 protease. Consumption of the intracellular Jiv pool is responsible for temporal regulation of protease activity: overexpression of Jiv interfered with regulation and correlated with increased accumulation of viral RNA; downregulation of the cellular Jiv level accelerated the decline of protease activity and reduced intracellular viral RNA levels and virion production. Accordingly, the amount of a cellular protein controls pestiviral replication by limiting the generation of active viral protease molecules and replication complexes. Importantly, this unique mechanism of replication control is essential for maintenance of the noncytopathogenic phenotype of the virus and thereby for its ability to establish persistent infections. These results add an entirely novel aspect to the understanding of the molecular basis of viral persistence.
Figures


References
-
- Beck, J., and M. Nassal. 2003. Efficient Hsp90-independent in vitro activation by Hsc70 and Hsp40 of duck hepatitis B virus reverse transcriptase, an assumed Hsp90 client protein. J. Biol. Chem. 278:36128-36138. - PubMed
-
- Bridge, A. J., S. Pebernard, A. Ducraux, A. L. Nicoulaz, and R. Iggo. 2003. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34:263-264. - PubMed
-
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

