Age-dependent role for CCR5 in antiviral host defense against herpes simplex virus type 2
- PMID: 16014944
- PMCID: PMC1181601
- DOI: 10.1128/JVI.79.15.9831-9841.2005
Age-dependent role for CCR5 in antiviral host defense against herpes simplex virus type 2
Abstract
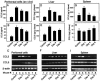
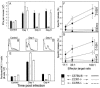
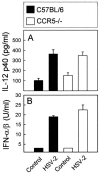
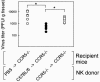
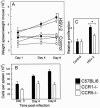
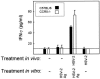
Elimination of viral infections is dependent on rapid recruitment and activation of leukocytes with antiviral activities to infected areas. Chemokines constitute a class of cytokines that have regulatory effects on leukocyte migration and activity. In this study we have studied the role of CC chemokine receptor 1 (CCR1) and CCR5 in host defense during a generalized herpes simplex virus type 2 (HSV-2) infection. Whereas both 4- and 8-week-old CCR1(-/-) mice resembled wild-type mice (C57BL/6) with respect to defense against the infection, significantly higher virus titers were seen in the livers and brains of 4-week-old CCR5(-/-) mice. At the age of 8 weeks, CCR5(-/-) were indistinguishable from wild-type mice and cleared the infection from liver and spleen. Although 4-week-old CCR5(-/-) mice were able to recruit natural killer (NK) cells to the site of infection, these cells had reduced cytotoxic activity compared to NK cells from wild-type mice. This was not due to lower production of alpha/beta interferon or interleukin-12, two well-described activators of cytotoxic activity in NK cells. We also noted that the spleens of young CCR5(-/-) mice did not increase in size during infection as did the spleens of wild-type and CCR1(-/-) mice. This observation was accompanied by impaired proliferation of CCR5(-/-) splenocytes (SCs) ex vivo. Moreover, migration of CD8(+) T cells to the liver in response to infection was impaired in CCR5(-/-) mice, and adoptive transfer of SCs from CCR5(-/-) mice infected for 6 days into newly infected wild-type mice did not improve antiviral activity in the liver, in contrast to what was seen in mice receiving immune SCs from wild-type mice. Altogether, this study shows that CCR5 plays an age-dependent role in host defense against HSV-2 by supporting both the innate and adaptive immune response.
Figures




References
-
- Aliberti, J., C. Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 α+ dendritic cells. Nat. Immunol. 1:83-87. - PubMed
-
- Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. - PubMed
-
- Cook, D. N., M. A. Beck, T. M. Coffman, S. L. Kirby, J. F. Sheridan, I. B. Pragnell, and O. Smithies. 1995. Requirement of MIP-1 α for an inflammatory response to viral infection. Science 269:1583-1585. - PubMed
-
- Croft, M. 2003. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3:609-620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

