Characterization of immune responses induced by intramuscular vaccination with DNA vaccines encoding measles virus hemagglutinin and/or fusion proteins
- PMID: 16014946
- PMCID: PMC1181616
- DOI: 10.1128/JVI.79.15.9854-9861.2005
Characterization of immune responses induced by intramuscular vaccination with DNA vaccines encoding measles virus hemagglutinin and/or fusion proteins
Abstract
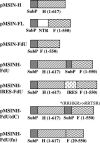
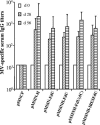
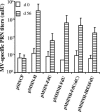
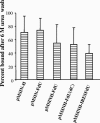
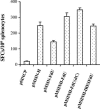
Measles virus (MV) hemagglutinin (MV-H) and fusion (MV-F) proteins induce plaque reduction neutralizing (PRN) antibodies and cell-mediated immune responses that protect against clinical measles. DNA vaccines that encode MV-H and MV-F are being investigated as a new generation of measles vaccine to protect infants too young to receive currently licensed attenuated measles vaccines. However, it is unclear whether DNA vaccines encoding both MV-H and MV-F act synergistically to induce stronger immunity than immunization with plasmids encoding MV-H or MV-F alone. To address this question, we generated Sindbis virus-based pSINCP DNA vaccines that encode either MV-H or MV-F alone or bicistronic or fusion system vectors that encode both MV-H and MV-F (to mimic MV infection where both MV-H and MV-F proteins are expressed by the same mammalian cell). Mice immunized with DNA vaccine encoding MV-H alone developed significantly greater PRN titers than mice immunized with bicistronic constructs. Interestingly, the presence of MV-F in the bicistronic constructs stimulated serum MV-specific immunoglobulin G of reduced avidity. By contrast, mice immunized with bicistronic constructs induced equivalent or higher levels of MV-specific gamma interferon responses than mice immunized with DNA vaccine encoding MV-H alone. These data will help guide the design of DNA-based MV vaccines to be used early in life in a heterologous prime-boost strategy.
Figures
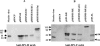

References
-
- Ad Hoc Group for the Study of Pertussis Vaccines. 1988. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet i:955-960. - PubMed
-
- Albrecht, P., F. A. Ennis, E. J. Saltzman, and S. Krugman. 1977. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J. Pediatr. 91:715-718. - PubMed
-
- Bosu, W. K., S. Odoom, P. Deiter, and M. Essel-Ahun. 2003. Epidemiology of measles in the central region of Ghana: a five-year case review in three district hospitals. East Afr. Med. J. 80:312-317. - PubMed
-
- Brodsky, A. L. 1972. Atypical measles. Severe illness in recipients of killed measles virus vaccine upon exposure to natural infection. JAMA 222:1415-1416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

