Herpes simplex virus type 1 strain HSV1716 grown in baby hamster kidney cells has altered tropism for nonpermissive Chinese hamster ovary cells compared to HSV1716 grown in vero cells
- PMID: 16014957
- PMCID: PMC1181565
- DOI: 10.1128/JVI.79.15.9970-9981.2005
Herpes simplex virus type 1 strain HSV1716 grown in baby hamster kidney cells has altered tropism for nonpermissive Chinese hamster ovary cells compared to HSV1716 grown in vero cells
Abstract
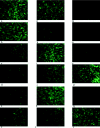
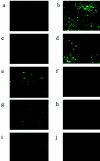
Chinese hamster ovary (CHO) cells are traditionally regarded as nonpermissive cells for herpes simplex virus type 1 (HSV-1) infection as they lack the specific entry receptors, and modified CHO cells have been instrumental in the identification of HSV-1 receptors in numerous studies. In this report we demonstrate that the HSV-1 strain 17+ variant HSV1716 is able to infect unmodified CHO cells but only if the virus is propagated in baby hamster kidney (BHK) cells. Infection of CHO cells by BHK-propagated HSV1716 results in expression of immediate-early, early, and late viral genes, and infectious progeny virions are produced. In normally cultured CHO cells, up to a maximum of 50% of cells were permissive for BHK-propagated HSV1716 infection, with 24 h of serum starvation increasing this to 100% of CHO cells, suggesting that the mechanism used by BHK-propagated virus to infect CHO cells was cell cycle dependent. The altered tropism of HSV1716 was also evident in another nonpermissive mouse melanoma cell line and is an exclusive property resulting from propagation of the virus using BHK cells, as viruses propagated on Vero, C8161 (a human melanoma cell line), or indeed, CHO cells were completely unable to infect either CHO or mouse melanoma cells.
Figures



Similar articles
-
A strategy for systemic delivery of the oncolytic herpes virus HSV1716: redirected tropism by antibody-binding sites incorporated on the virion surface as a glycoprotein D fusion protein.Gene Ther. 2008 Dec;15(24):1579-92. doi: 10.1038/gt.2008.121. Epub 2008 Aug 14. Gene Ther. 2008. PMID: 18701918
-
A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus.Virology. 1998 Jun 20;246(1):179-89. doi: 10.1006/viro.1998.9218. Virology. 1998. PMID: 9657005
-
Functional characterization of the HveA homolog specified by African green monkey kidney cells with a herpes simplex virus expressing the green fluorescence protein.Virology. 1999 Jun 5;258(2):365-74. doi: 10.1006/viro.1999.9743. Virology. 1999. PMID: 10366573
-
[Modification of Chinese hamster ovary cells].Sheng Wu Gong Cheng Xue Bao. 2002 Jul;18(4):415-9. Sheng Wu Gong Cheng Xue Bao. 2002. PMID: 12385235 Review. Chinese.
-
Problems with BHK 21 cells.Dev Biol Stand. 1998;93:85-8. Dev Biol Stand. 1998. PMID: 9737382 Review.
Cited by
-
Virus susceptibility of Chinese hamster ovary (CHO) cells and detection of viral contaminations by adventitious agent testing.Biotechnol Bioeng. 2010 Jul 1;106(4):598-607. doi: 10.1002/bit.22723. Biotechnol Bioeng. 2010. PMID: 20503298 Free PMC article.
-
Exploiting non-permissive CHO cells as a rapid and efficient method for recombinant HSV-1 isolation.AMB Express. 2024 May 9;14(1):53. doi: 10.1186/s13568-024-01709-0. AMB Express. 2024. PMID: 38722404 Free PMC article.
-
Primate Simplexviruses Differ in Tropism for Macaque Cells.Microorganisms. 2022 Dec 21;11(1):26. doi: 10.3390/microorganisms11010026. Microorganisms. 2022. PMID: 36677317 Free PMC article.
-
Herpes simplex virus internalization into epithelial cells requires Na+/H+ exchangers and p21-activated kinases but neither clathrin- nor caveolin-mediated endocytosis.J Virol. 2014 Nov;88(22):13378-95. doi: 10.1128/JVI.03631-13. Epub 2014 Sep 10. J Virol. 2014. PMID: 25210183 Free PMC article.
-
Molecular cloning and host range analysis of three cytomegaloviruses from Mastomys natalensis.J Virol. 2025 May 20;99(5):e0214724. doi: 10.1128/jvi.02147-24. Epub 2025 Apr 9. J Virol. 2025. PMID: 40202317 Free PMC article.
References
-
- Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. - PubMed
-
- Conner, J., J. Furlong, J. Murray, M. Meighan, A. Cross, H. Marsden, and J. B. Clements. 1993. Herpes simplex virus type 1 ribonucleotide reductase large subunit: regions of the protein essential for subunit interaction and dimerization. Biochemistry 32:13673-13680. - PubMed
-
- Davison, A. J., and J. B. Clements. 1997. Herpesviruses: general properties, p. 309. In B. W. J. Mahy and L. H. Collier (ed.), Topley and Wilsons' principles of bacteriology virology and immunology. Edward Arnold, London, United Kingdom.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

