Molecular basis for mitochondrial localization of viral particles during beet necrotic yellow vein virus infection
- PMID: 16014959
- PMCID: PMC1181617
- DOI: 10.1128/JVI.79.15.9991-10002.2005
Molecular basis for mitochondrial localization of viral particles during beet necrotic yellow vein virus infection
Abstract
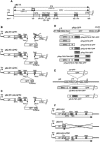
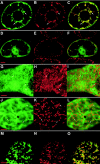
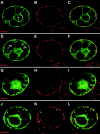
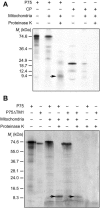
During infection, Beet necrotic yellow vein virus (BNYVV) particles localize transiently to the cytosolic surfaces of mitochondria. To understand the molecular basis and significance of this localization, we analyzed the targeting and membrane insertion properties of the viral proteins. ORF1 of BNYVV RNA-2 encodes the 21-kDa major coat protein, while ORF2 codes for a 75-kDa minor coat protein (P75) by readthrough of the ORF1 stop codon. Bioinformatic analysis highlighted a putative mitochondrial targeting sequence (MTS) as well as a major (TM1) and two minor (TM3 and TM4) transmembrane regions in the N-terminal part of the P75 readthrough domain. Deletion and gain-of-function analyses based on the localization of green fluorescent protein (GFP) fusions showed that the MTS was able to direct a reporter protein to mitochondria but that the protein was not persistently anchored to the organelles. GFP fused either to MTS and TM1 or to MTS and TM3-TM4 efficiently and specifically associated with mitochondria in vivo. The actual role of the individual domains in the interaction with the mitochondria seemed to be determined by the folding of P75. Anchoring assays to the outer membranes of isolated mitochondria, together with in vivo data, suggest that the TM3-TM4 domain is the membrane anchor in the context of full-length P75. All of the domains involved in mitochondrial targeting and anchoring were also indispensable for encapsidation, suggesting that the assembly of BNYVV particles occurs on mitochondria. Further data show that virions are subsequently released from mitochondria and accumulate in the cytosol.
Figures
References
-
- Adams, M. J., J. F. Antoniw, and J. G. Mullins. 2001. Plant virus transmission by plasmodiophorid fungi is associated with distinctive transmembrane regions of virus-encoded proteins. Arch. Virol. 146:1139-1153. - PubMed
-
- Ahting, U., T. Waizenegger, W. Neupert, and D. Rapaport. 2005. Signal-anchored proteins follow a unique insertion pathway into the outer membrane of mitochondria. J. Biol. Chem. 280:48-53. - PubMed
-
- Bleykasten, C., D. Gilmer, H. Guilley, K. E. Richards, and G. Jonard. 1996. Beet necrotic yellow vein virus 42 kDa triple gene block protein binds nucleic acid in vitro. J. Gen. Virol. 77:889-897. - PubMed
-
- Bouzoubaa, S., L. Quillet, H. Guilley, G. Jonard, and K. Richards. 1987. Nucleotide sequence of Beet necrotic yellow vein virus RNA-1. J. Gen. Virol. 68:615-626.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

