The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35
- PMID: 16014961
- PMCID: PMC1181579
- DOI: 10.1128/JVI.79.15.10013-10022.2005
The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35
Abstract
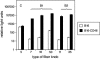
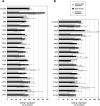
The human regulator of complement activation membrane cofactor protein (CD46) has recently been identified as an attachment receptor for most species B adenoviruses (Ads), including Ad type 3 (Ad3), Ad11, and Ad35, as well as species D Ad37. To characterize the interaction between Ad35 and CD46, hybrid receptors composed of different CD46 short consensus repeat (SCR) domains fused to immunoglobulin-like domains of CD4 and a set of 36 CD46 mutants containing semiconservative changes of single amino acids within SCR domains I and II were tested in binding and in Ad35-mediated luciferase transduction assays. In addition, anti-CD46 antibodies and soluble polypeptides constituting various CD46 domains were used in binding inhibition studies. Our data indicate that (i) CD46 SCR I or SCR II alone confers low but significant Ad35 binding; (ii) the presence of SCR I and II is required for optimal binding and transgene expression; (iii) transduction efficiencies equivalent to that of full-length CD46 are obtained if SCR I and II are at an appropriate distance from the cell membrane; (iv) ablation of the N-glycan attached to SCR I has no influence on receptor function, whereas ablation of the SCR II N-glycan results in about a two- to threefold reduction of binding and transgene expression; (v) most putative Ad35 binding residues are located on the same solvent-exposed face of the SCR I or SCR II domain, which are twisted by about 90 degrees ; and (vi) the putative Ad35 binding sites partly overlap with the measles virus binding surface.
Figures



Similar articles
-
Localization of regions in CD46 that interact with adenovirus.J Virol. 2005 Jun;79(12):7503-13. doi: 10.1128/JVI.79.12.7503-7513.2005. J Virol. 2005. PMID: 15919905 Free PMC article.
-
Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor.J Gen Virol. 2007 Nov;88(Pt 11):2925-2934. doi: 10.1099/vir.0.83142-0. J Gen Virol. 2007. PMID: 17947513
-
The short consensus repeats 1 and 2, not the cytoplasmic domain, of human CD46 are crucial for infection of subgroup B adenovirus serotype 35.J Control Release. 2006 Jul 20;113(3):271-8. doi: 10.1016/j.jconrel.2006.05.007. Epub 2006 Jun 21. J Control Release. 2006. PMID: 16790289
-
CD46 (membrane cofactor protein of complement, measles virus receptor): structural and functional divergence among species (review).Int J Mol Med. 1998 May;1(5):809-16. doi: 10.3892/ijmm.1.5.809. Int J Mol Med. 1998. PMID: 9852300 Review.
-
Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster.Annu Rev Immunol. 1991;9:431-55. doi: 10.1146/annurev.iy.09.040191.002243. Annu Rev Immunol. 1991. PMID: 1910685 Review.
Cited by
-
Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation.Proc Natl Acad Sci U S A. 2011 May 3;108(18):7499-504. doi: 10.1073/pnas.1017146108. Epub 2011 Apr 18. Proc Natl Acad Sci U S A. 2011. PMID: 21502499 Free PMC article.
-
Advanced generation adenoviral virotherapy agents embody enhanced potency based upon CAR-independent tropism.Clin Cancer Res. 2006 May 1;12(9):2651-6. doi: 10.1158/1078-0432.CCR-06-0497. Clin Cancer Res. 2006. PMID: 16675555 Free PMC article. Review. No abstract available.
-
Electrostatic Interactions between Complement Regulator CD46(SCR1-2) and Adenovirus Ad11/Ad21 Fiber Protein Knob.Mol Biol Int. 2015;2015:967465. doi: 10.1155/2015/967465. Epub 2015 Aug 19. Mol Biol Int. 2015. PMID: 26357573 Free PMC article.
-
The RGD-binding integrins αvβ6 and αvβ8 are receptors for mouse adenovirus-1 and -3 infection.PLoS Pathog. 2021 Dec 15;17(12):e1010083. doi: 10.1371/journal.ppat.1010083. eCollection 2021 Dec. PLoS Pathog. 2021. PMID: 34910784 Free PMC article.
-
Glycomics and Proteomics Approaches to Investigate Early Adenovirus-Host Cell Interactions.J Mol Biol. 2018 Jun 22;430(13):1863-1882. doi: 10.1016/j.jmb.2018.04.039. Epub 2018 May 7. J Mol Biol. 2018. PMID: 29746851 Free PMC article. Review.
References
-
- Adams, E. M., M. C. Brown, M. Nunge, M. Krych, and J. P. Atkinson. 1991. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J. Immunol. 147:3005-3011. - PubMed
-
- Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. - PubMed
-
- Bork, P., A. K. Downing, B. Kieffer, and I. D. Campbell. 1996. Structure and distribution of modules in extracellular proteins. Q. Rev. Biophys. 29:119-167. - PubMed
-
- Buchholz, C. J., D. Koller, P. Devaux, C. Mumenthaler, J. Schneider-Schaulies, W. Braun, D. Gerlier, and R. Cattaneo. 1997. Mapping of the primary binding site of measles virus to its receptor CD46. J. Biol. Chem. 272:22072-22079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

