Phosphorylation of bluetongue virus nonstructural protein 2 is essential for formation of viral inclusion bodies
- PMID: 16014962
- PMCID: PMC1181561
- DOI: 10.1128/JVI.79.15.10023-10031.2005
Phosphorylation of bluetongue virus nonstructural protein 2 is essential for formation of viral inclusion bodies
Abstract
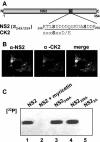
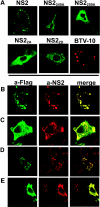
In bluetongue virus (BTV)-infected cells, large cytoplasmic aggregates are formed, termed viral inclusion bodies (VIBs), which are believed to be the sites of viral replication and morphogenesis. The BTV nonstructural protein NS2 is the major component of VIBs. NS2 undergoes intracellular phosphorylation and possesses a strong single-stranded RNA binding activity. By changing phosphorylated amino acids to alanines and aspartates, we have mapped the phosphorylated sites of NS2 to two serine residues at positions 249 and 259. Since both of these serines are within the context of protein kinase CK2 recognition signals, we have further examined if CK2 is involved in NS2 phosphorylation by both intracellular colocalization and an in vitro phosphorylation assay. In addition, we have utilized the NS2 mutants to determine the role of phosphorylation on NS2 activities. The data obtained demonstrate that NS2 phosphorylation is not necessary either for its RNA binding properties or for its ability to interact with the viral polymerase VP1. However, phosphorylated NS2 exhibited VIB formation while unmodified NS2 failed to assemble as VIBs although smaller oligomeric forms of NS2 were readily formed. Our data reveal that NS2 phosphorylation controls VIBs formation consistent with a model in which NS2 provides the matrix for viral assembly.
Figures



Similar articles
-
Cellular Casein Kinase 2 and Protein Phosphatase 2A Modulate Replication Site Assembly of Bluetongue Virus.J Biol Chem. 2016 Jul 8;291(28):14566-74. doi: 10.1074/jbc.M116.714766. Epub 2016 May 16. J Biol Chem. 2016. PMID: 27226558 Free PMC article.
-
A Calcium Sensor Discovered in Bluetongue Virus Nonstructural Protein 2 Is Critical for Virus Replication.J Virol. 2020 Sep 29;94(20):e01099-20. doi: 10.1128/JVI.01099-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32759321 Free PMC article.
-
Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly.BMC Mol Biol. 2007 Jan 22;8:4. doi: 10.1186/1471-2199-8-4. BMC Mol Biol. 2007. PMID: 17241458 Free PMC article.
-
Insights into the role of the non-structural protein 2 (NS2) in Bluetongue virus morphogenesis.Virus Res. 2010 Aug;151(2):109-17. doi: 10.1016/j.virusres.2010.05.014. Virus Res. 2010. PMID: 20621672 Review.
-
Nonstructural proteins involved in genome packaging and replication of rotaviruses and other members of the Reoviridae.Virus Res. 2004 Apr;101(1):57-66. doi: 10.1016/j.virusres.2003.12.006. Virus Res. 2004. PMID: 15010217 Review.
Cited by
-
Cellular Casein Kinase 2 and Protein Phosphatase 2A Modulate Replication Site Assembly of Bluetongue Virus.J Biol Chem. 2016 Jul 8;291(28):14566-74. doi: 10.1074/jbc.M116.714766. Epub 2016 May 16. J Biol Chem. 2016. PMID: 27226558 Free PMC article.
-
Bluetongue virus entry into cells.J Virol. 2007 May;81(9):4819-27. doi: 10.1128/JVI.02284-06. Epub 2007 Jan 31. J Virol. 2007. PMID: 17267479 Free PMC article.
-
Molecular Characterization of Outer Capsid Proteins VP5 and VP7 of Grass Carp Reovirus.Viruses. 2022 May 12;14(5):1032. doi: 10.3390/v14051032. Viruses. 2022. PMID: 35632773 Free PMC article.
-
Bluetongue virus assembly and exit pathways.Adv Virus Res. 2020;108:249-273. doi: 10.1016/bs.aivir.2020.08.002. Epub 2020 Sep 16. Adv Virus Res. 2020. PMID: 33837718 Free PMC article. Review.
-
Phosphoproteomic Analysis Reveals the Importance of Kinase Regulation During Orbivirus Infection.Mol Cell Proteomics. 2017 Nov;16(11):1990-2005. doi: 10.1074/mcp.M117.067355. Epub 2017 Aug 29. Mol Cell Proteomics. 2017. PMID: 28851738 Free PMC article.
References
-
- Afrikanova, I., M. C. Miozzo, S. Giambiagi, and O. Burrone. 1996. Phosphorylation generates different forms of rotavirus NSP5. J. Gen. Virol. 77:2059-2065. - PubMed
-
- Blom, N., S. Gammeltoft, and S. Brunak. 1999. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351-1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

