Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF-1
- PMID: 16014964
- PMCID: PMC1181586
- DOI: 10.1128/JVI.79.15.10040-10052.2005
Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF-1
Abstract
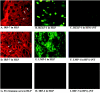
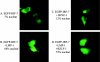
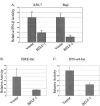
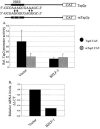
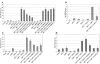
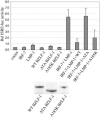
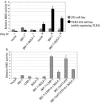
Virus infection stimulates potent antiviral responses; specifically, Epstein-Barr virus (EBV) infection induces and activates interferon regulatory factor 7 (IRF-7), which is essential for production of alpha/beta interferons (IFN-alpha/beta) and upregulates expression of Tap-2. Here we present evidence that during cytolytic viral replication the immediate-early EBV protein BZLF-1 counteracts effects of IRF-7 that are central to host antiviral responses. We initiated these studies by examining IRF-7 protein expression in vivo in lesions of hairy leukoplakia (HLP) in which there is abundant EBV replication but the expected inflammatory infiltrate is absent. This absence might predict that factors involved in the antiviral response are absent or inactive. First, we detected significant levels of IRF-7 in the nucleus, as well as in the cytoplasm, of cells in HLP lesions. IRF-7 activity in cell lines during cytolytic viral replication was examined by assay of the IRF-7-responsive promoters, IFN-alpha4, IFN-beta, and Tap-2, as well as of an IFN-stimulated response element (ISRE)-containing reporter construct. These reporter constructs showed consistent reduction of activity during lytic replication. Both endogenous and transiently expressed IRF-7 and EBV BZLF-1 proteins physically associate in cell culture, although BZLF-1 had no effect on the nuclear localization of IRF-7. However, IRF-7-dependent activity of the IFN-alpha4, IFN-beta, and Tap-2 promoters, as well as an ISRE promoter construct, was inhibited by BZLF-1. This inhibition occurred in the absence of other EBV proteins and was independent of IFN signaling. Expression of BZLF-1 also inhibited activation of IRF-7 by double-stranded RNA, as well as the activity of a constitutively active mutant form of IRF-7. Negative regulation of IRF-7 by BZLF-1 required the activation domain but not the DNA-binding domain of BZLF-1. Thus, EBV may subvert cellular antiviral responses and immune detection by blocking the activation of IFN-alpha4, IFN-beta, and Tap-2 by IRF-7 through the medium of BZLF-1 as a negative regulator.
Figures

Similar articles
-
Interferon regulatory factor 7 mediates activation of Tap-2 by Epstein-Barr virus latent membrane protein 1.J Virol. 2001 Jan;75(1):341-50. doi: 10.1128/JVI.75.1.341-350.2001. J Virol. 2001. PMID: 11119603 Free PMC article.
-
Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1.J Virol. 2000 Feb;74(3):1061-8. doi: 10.1128/jvi.74.3.1061-1068.2000. J Virol. 2000. PMID: 10627515 Free PMC article.
-
Latency of Epstein-Barr virus is stabilized by antisense-mediated control of the viral immediate-early gene BZLF-1.J Med Virol. 1999 Dec;59(4):512-9. doi: 10.1002/(sici)1096-9071(199912)59:4<512::aid-jmv15>3.0.co;2-b. J Med Virol. 1999. PMID: 10534735
-
On the role of IRF in host defense.J Interferon Cytokine Res. 2002 Jan;22(1):59-71. doi: 10.1089/107999002753452665. J Interferon Cytokine Res. 2002. PMID: 11846976 Review.
-
The control of lytic replication of Epstein-Barr virus in B lymphocytes (Review).Int J Mol Med. 1998 Jan;1(1):137-42. doi: 10.3892/ijmm.1.1.137. Int J Mol Med. 1998. PMID: 9852211 Review.
Cited by
-
Epstein-Barr Virus (EBV) Genotypes Associated with the Immunopathological Profile of People Living with HIV-1: Immunological Aspects of Primary EBV Infection.Viruses. 2022 Jan 18;14(2):168. doi: 10.3390/v14020168. Viruses. 2022. PMID: 35215762 Free PMC article.
-
Innate immune modulation in EBV infection.Herpesviridae. 2011 Jan 5;2(1):1. doi: 10.1186/2042-4280-2-1. Herpesviridae. 2011. PMID: 21429244 Free PMC article.
-
The Epstein-Barr virus BZLF1 protein inhibits tumor necrosis factor receptor 1 expression through effects on cellular C/EBP proteins.J Virol. 2010 Dec;84(23):12362-74. doi: 10.1128/JVI.00712-10. Epub 2010 Sep 22. J Virol. 2010. PMID: 20861254 Free PMC article.
-
Multiple sclerosis and infection: history, EBV, and the search for mechanism.Microbiol Mol Biol Rev. 2025 Mar 27;89(1):e0011923. doi: 10.1128/mmbr.00119-23. Epub 2025 Jan 16. Microbiol Mol Biol Rev. 2025. PMID: 39817754 Review.
-
Evasion of adaptive and innate immune response mechanisms by γ-herpesviruses.Curr Opin Virol. 2013 Jun;3(3):285-95. doi: 10.1016/j.coviro.2013.05.011. Epub 2013 Jun 2. Curr Opin Virol. 2013. PMID: 23735334 Free PMC article. Review.
References
-
- Abele, R., and R. Tampe. 2004. The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology 19:216-224. - PubMed
-
- Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. - PMC - PubMed
-
- Allavena, P., L. Piemonti, D. Longoni, S. Bernasconi, A. Stoppacciaro, L. Ruco, and A. Mantovani. 1998. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 28:359-369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

