Identification of two new HLA-A*1101-restricted tax epitopes recognized by cytotoxic T lymphocytes in an adult T-cell leukemia patient after hematopoietic stem cell transplantation
- PMID: 16014972
- PMCID: PMC1181560
- DOI: 10.1128/JVI.79.15.10088-10092.2005
Identification of two new HLA-A*1101-restricted tax epitopes recognized by cytotoxic T lymphocytes in an adult T-cell leukemia patient after hematopoietic stem cell transplantation
Abstract
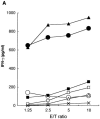
We previously reported that Tax-specific CD8(+) cytotoxic T lymphocytes (CTLs), directed to single epitopes restricted by HLA-A2 or A24, expanded in vitro and in vivo in peripheral blood mononuclear cells (PBMC) from some adult T-cell leukemia (ATL) patients after but not before allogeneic hematopoietic stem cell transplantation (HSCT). Here, we demonstrated similar Tax-specific CTL expansion in PBMC from another post-HSCT ATL patient without HLA-A2 or A24, whose CTLs equally recognized two newly identified epitopes, Tax88-96 and Tax272-280, restricted by HLA-A11, suggesting that these immunodominant Tax epitopes are present in the ATL patient in vivo.
Figures






References
-
- Bieganowska, K., P. Hollsberg, G. J. Buckle, D. G. Lim, T. F. Greten, J. Schneck, J. D. Altman, S. Jacobson, S. L. Ledis, B. Hanchard, J. Chin, O. Morgan, P. A. Roth, and D. A. Hafler. 1999. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11-19 tetramer complexes in patients with human T cell lymphotropic virus-associated myelopathy. J. Immunol. 162:1765-1771. - PubMed
-
- Gomi, S., M. Nakao, F. Niiya, Y. Imamura, K. Kawano, S. Nishizaka, A. Hayashi, Y. Sobao, K. Oizumi, and K. Itoh. 1999. A cyclophilin B gene encodes antigenic epitopes recognized by HLA-A24-restricted and tumor-specific CTLs. J. Immunol. 163:4994-5004. - PubMed
-
- Goulmy, E., R. Schipper, J. Pool, E. Blokland, J. H. Falkenburg, J. Vossen, A. Grathwohl, G. B. Vogelsang, H. C. van Houwelingen, and J. J. van Rood. 1996. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N. Engl. J. Med. 334:281-285. - PubMed
-
- Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, A. Hasegawa, F. Takemura, T. Masuda, and M. Kannagi. 2001. Regression of human T-cell leukemia virus type I (HTLV-I)-associated lymphomas in a rat model: peptide-induced T-cell immunity. J. Natl. Cancer Inst. 93:1775-1783. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

