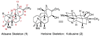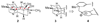Enantioselective approach to the hetisine alkaloids. Synthesis of the 3-methyl-1-aza-tricyclo[5.2.1.0(3,8)]decane core via intramolecular dipolar cycloaddition
- PMID: 16018651
- PMCID: PMC2593868
- DOI: 10.1021/ol051184v
Enantioselective approach to the hetisine alkaloids. Synthesis of the 3-methyl-1-aza-tricyclo[5.2.1.0(3,8)]decane core via intramolecular dipolar cycloaddition
Abstract
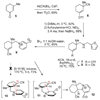
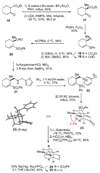
[structure: see text]. An efficient, enantioselective approach to the hetisine class of the C(20)-diterpenoid alkaloids is described. The strategy involves an intramolecular oxidopyridinium dipolar cycloaddition as the key transformation, in which simultaneous formation of the C5-C6 and C10-C20 bonds in the 3-methyl-1-aza-tricyclo[5.2.1.0(3,8)]decane core of the hetisine alkaloids is effected.
Figures
Similar articles
-
Efficient synthetic access to the hetisine C20-diterpenoid alkaloids. A concise synthesis of nominine via oxidoisoquinolinium-1,3-dipolar and dienamine-Diels-Alder cycloadditions.J Am Chem Soc. 2006 Jul 12;128(27):8734-5. doi: 10.1021/ja0625430. J Am Chem Soc. 2006. PMID: 16819859 Free PMC article.
-
Asymmetric synthetic access to the hetisine alkaloids: total synthesis of (+)-nominine.Chemistry. 2008;14(5):1654-65. doi: 10.1002/chem.200701290. Chemistry. 2008. PMID: 18046691 Free PMC article.
-
Synthesis of Three-Dimensionally Fascinating Diterpenoid Alkaloids and Related Diterpenes.Acc Chem Res. 2021 Jan 5;54(1):22-34. doi: 10.1021/acs.accounts.0c00720. Epub 2020 Dec 22. Acc Chem Res. 2021. PMID: 33351595
-
A Case for Bond-Network Analysis in the Synthesis of Bridged Polycyclic Complex Molecules: Hetidine and Hetisine Diterpenoid Alkaloids.Angew Chem Int Ed Engl. 2020 Jun 26;59(27):10722-10731. doi: 10.1002/anie.201909656. Epub 2020 Apr 15. Angew Chem Int Ed Engl. 2020. PMID: 31808282 Free PMC article. Review.
-
[Research progress on C_(20)-diterpenoid alkaloids and their bioactivities in Ranunculaceae].Zhongguo Zhong Yao Za Zhi. 2024 Aug;49(15):4054-4068. doi: 10.19540/j.cnki.cjcmm.20240410.202. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39307757 Review. Chinese.
Cited by
-
Efficient synthetic access to the hetisine C20-diterpenoid alkaloids. A concise synthesis of nominine via oxidoisoquinolinium-1,3-dipolar and dienamine-Diels-Alder cycloadditions.J Am Chem Soc. 2006 Jul 12;128(27):8734-5. doi: 10.1021/ja0625430. J Am Chem Soc. 2006. PMID: 16819859 Free PMC article.
-
Gallium(III)-catalyzed cycloisomerization approach to the diterpenoid alkaloids: construction of the core structure for the hetidines and hetisines.Angew Chem Int Ed Engl. 2013 Apr 26;52(18):4854-7. doi: 10.1002/anie.201209030. Epub 2013 Mar 26. Angew Chem Int Ed Engl. 2013. PMID: 23533012 Free PMC article.
-
(4+3) cycloaddition reactions of nitrogen-stabilized oxyallyl cations.Chemistry. 2011 Mar 28;17(14):3812-22. doi: 10.1002/chem.201100260. Epub 2011 Mar 7. Chemistry. 2011. PMID: 21384451 Free PMC article.
-
Developing a diastereoselective intramolecular [4+3] cycloaddition of nitrogen-stabilized oxyallyl cations derived from N-sulfonyl-substituted allenamides.J Org Chem. 2011 May 6;76(9):3246-57. doi: 10.1021/jo200147h. Epub 2011 Apr 8. J Org Chem. 2011. PMID: 21449577 Free PMC article.
-
Introduction of the (-)-berkelic acid side chain and assignment of the C-22 stereochemistry.J Org Chem. 2009 Aug 21;74(16):6245-52. doi: 10.1021/jo901221a. J Org Chem. 2009. PMID: 19630376 Free PMC article.
References
-
- Wang F-P, Liang X-T. In: The Alkaloids. Cordell GA, editor. Vol. 59. San Diego, CA: Academic Press; 2002. pp. 1–280. - PubMed
-
- Suginome H, Shimanouti F. Justus Liebigs Ann. Chem. 1940;545:220–228.
-
- Okamoto T. Chem. Pharm. Bull. 1959;7:44–49.
- Pelletier SW, Wright LH, Newton MG, Wright H. J. Chem. Soc., Chem. Commun. 1970:98–99.
- Sakai S, Yamamoto I, Yamaguchi K, Takayama H, Ito M, Okamoto T. Chem. Pharm. Bull. 1982;30:4579–4584.
- Ulubelen A, Desai HK, Srivastava SK, Hart BP, Park J, Joshi BS, Pelletier SW, Mericli AH, Mericli F, Ilarslan R. J. Nat. Prod. 1996;59:360–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous