Incidence and risk factors for liver enzyme elevation during highly active antiretroviral therapy in HIV-HCV co-infected patients: results from the Italian EPOKA-MASTER Cohort
- PMID: 16018804
- PMCID: PMC1188059
- DOI: 10.1186/1471-2334-5-58
Incidence and risk factors for liver enzyme elevation during highly active antiretroviral therapy in HIV-HCV co-infected patients: results from the Italian EPOKA-MASTER Cohort
Abstract
Background: The risk of hepatotoxicity associated with different highly active antiretroviral therapy (HAART) regimens (containing multiple-protease inhibitors, single-protease inhibitors or non nucleoside reverse transcriptase inhibitors) in HIV-HCV co-infected patients has not been fully assessed.
Methods: Retrospective analysis of a prospective cohort of 1,038 HIV-HCV co-infected patients who commenced a new HAART in the Italian MASTER database. Patients were stratified into naïve and experienced to antiretroviral therapy before starting the study regimens. Time to grade > or =III hepatotoxicity (as by ACTG classification) was the primary outcome. Secondary outcome was time to grade IV hepatotoxicity.
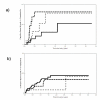
Results: Incidence of grade > or =III hepatotoxicity was 17.71 per 100 patient-years (p-yr) of follow up in naïve patient group and 8.22 per 100 p-yrs in experienced group (grade IV: 4.13 per 100 p-yrs and 1.08 per 100 p-yrs, respectively). In the latter group, the only independent factors associated with shorter time to the event at proportional hazards regression model were: previous liver transaminase elevations to grade > or =III, higher baseline alanine amino-transferase values, and use of a non nucleoside reverse transcriptase inhibitor based regimen. In the naive group, baseline aspartate transaminase level was associated with the primary outcome.
Conclusion: Use of a single or multiple protease inhibitor based regimen was not associated with risk of hepatotoxicity in either naïve or experienced patient groups to a statistically significant extent. A cautious approach with strict monitoring should be applied in HIV-HCV co-infected experienced patients with previous liver transaminase elevations, higher baseline alanine amino-transferase values and who receive regimens containing non nucleoside reverse transcriptase inhibitors.
Figures

References
-
- den Brinker M, Wit FW, Wertheim-van Dillen PM, Jurriaans S, Weel J, van Leeuwen R, Pakker NG, Reiss P, Danner SA, Weverling GJ, Lange JM. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14:2895–2902. doi: 10.1097/00002030-200012220-00011. - DOI - PubMed
-
- Martinez E, Blanco JL, Arnaiz JA, Perez-Cuevas JB, Mocroft A, Cruceta A, Marcos MA, Milinkovic A, Garcia-Viejo MA, Mallolas J, Carne X, Phillips A, Gatell JM. Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy. AIDS. 2001;15:1261–1268. doi: 10.1097/00002030-200107060-00007. - DOI - PubMed
-
- Monforte AA, Bugarini R, Pezzotti P, De LA, Antinori A, Mussini C, Vigevani GM, Tirelli U, Bruno R, Gritti F, Piazza M, Chigiotti S, Chirianni A, De SC, Pizzigallo E, Perrella O, Moroni M. Low frequency of severe hepatotoxicity and association with HCV coinfection in HIV-positive patients treated with HAART. J Acquir Immune Defic Syndr. 2001;28:114–123. doi: 10.1097/00126334-200110010-00002. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

