Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets
- PMID: 16020504
- PMCID: PMC1895051
- DOI: 10.1182/blood-2005-05-1933
Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets
Abstract
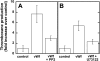
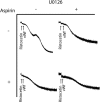
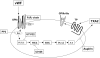
The binding of von Willebrand factor (VWF) to the platelet membrane glycoprotein Ib-IX (GPIb-IX) results in platelet activation. In this study, we sought to clarify previous conflicting reports and to elucidate the mechanism of activation and the precise role of extracellular signal-regulated kinase (Erk) in VWF-induced platelet activation. Erk2 is activated in platelets on stimulation with VWF/ristocetin in a time-dependent manner. VWF-induced Erk2 phosphorylation and thromboxane A2 (TXA2) release were completely blocked by PP2, an Src family kinase inhibitor, suggesting that Erk is downstream of Src family kinases. U73122, a phospholipase C inhibitor, also abolished TXA2 generation and Erk phosphorylation. Although VWF fostered the agglutination of platelets regardless of any additional treatment, the inhibition of mitogen-activated protein kinase kinase (MEK) with U0126 abolished VWF-induced platelet aggregation and thromboxane production in non-aspirin-treated washed platelets. However, in platelets treated with aspirin, VWF failed to cause any aggregation. Thus, we conclude that VWF stimulation of platelets results in phospholipase A2 activation through Erk stimulation and that Src family kinases and phospholipase C play essential roles in this event. We further conclude that VWF-induced platelet aggregation does not directly depend on Erk activation but has an absolute requirement for Src/Erk-mediated TXA2 generation.
Figures



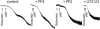
Comment in
-
Are Erk, Btk, and PECAM-1 major players in GPIb signaling? The challenge of unraveling signaling events downstream of platelet GPIb.Blood. 2007 Jan 15;109(2):846-7; discussion 847-8. doi: 10.1182/blood-2006-08-042036. Blood. 2007. PMID: 17210869 No abstract available.
Similar articles
-
Botrocetin/VWF-induced signaling through GPIb-IX-V produces TxA2 in an alphaIIbbeta3- and aggregation-independent manner.Blood. 2005 Oct 15;106(8):2750-6. doi: 10.1182/blood-2005-04-1667. Epub 2005 Jun 28. Blood. 2005. PMID: 15985541 Free PMC article.
-
Von Willebrand factor activates endothelial nitric oxide synthase in blood platelets by a glycoprotein Ib-dependent mechanism.J Thromb Haemost. 2006 Dec;4(12):2636-44. doi: 10.1111/j.1538-7836.2006.02195.x. J Thromb Haemost. 2006. PMID: 17100655
-
The role of Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation.Arterioscler Thromb Vasc Biol. 2012 Nov;32(11):2761-8. doi: 10.1161/ATVBAHA.112.254920. Epub 2012 Sep 20. Arterioscler Thromb Vasc Biol. 2012. PMID: 22995516 Free PMC article.
-
Syk Activity Is Dispensable for Platelet GP1b-IX-V Signaling.Int J Mol Sci. 2017 Jun 9;18(6):1238. doi: 10.3390/ijms18061238. Int J Mol Sci. 2017. PMID: 28598382 Free PMC article. Review.
-
Signaling mechanisms of the platelet glycoprotein Ib-IX complex.Platelets. 2022 Aug 18;33(6):823-832. doi: 10.1080/09537104.2022.2071852. Epub 2022 May 26. Platelets. 2022. PMID: 35615944 Free PMC article. Review.
Cited by
-
P2Y12 receptor: platelet thrombus formation and medical interventions.Int J Hematol. 2012 Nov;96(5):572-87. doi: 10.1007/s12185-012-1188-5. Epub 2012 Oct 1. Int J Hematol. 2012. PMID: 23054651 Review.
-
Thrombin and vascular inflammation.Mol Cell Biochem. 2012 Jan;359(1-2):301-13. doi: 10.1007/s11010-011-1024-x. Epub 2011 Aug 23. Mol Cell Biochem. 2012. PMID: 21858738 Review.
-
Proteomics of arterial thrombi in acute limb ischemia.J Thromb Thrombolysis. 2025 Jun 22. doi: 10.1007/s11239-025-03123-0. Online ahead of print. J Thromb Thrombolysis. 2025. PMID: 40544393
-
The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation.Blood. 2008 Jan 15;111(2):658-65. doi: 10.1182/blood-2007-04-085514. Epub 2007 Oct 3. Blood. 2008. PMID: 17914025 Free PMC article.
-
Comprehensive overview of COVID-19-related respiratory failure: focus on cellular interactions.Cell Mol Biol Lett. 2022 Jul 30;27(1):63. doi: 10.1186/s11658-022-00363-3. Cell Mol Biol Lett. 2022. PMID: 35907817 Free PMC article. Review.
References
-
- Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91: 2645-2657. - PubMed
-
- Packham MA. Role of platelets in thrombosis and hemostasis. Can J Physiol Pharmacol. 1994;72: 278-284. - PubMed
-
- Brass LF, Manning DR, Cichowski K, Abrams CS. Signaling through G proteins in platelets: to the integrins and beyond. Thromb Haemost. 1997;78: 581-589. - PubMed
-
- McNicol A, Israels SJ. Platelets and anti-platelet therapy. J Pharmacol Sci. 2003;93: 381-396. - PubMed
-
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8: 1227-1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

