Paramyosin phosphorylation site disruption affects indirect flight muscle stiffness and power generation in Drosophila melanogaster
- PMID: 16020538
- PMCID: PMC1180758
- DOI: 10.1073/pnas.0500945102
Paramyosin phosphorylation site disruption affects indirect flight muscle stiffness and power generation in Drosophila melanogaster
Abstract
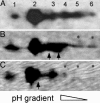
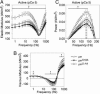
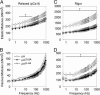
The phosphoprotein paramyosin is a major structural component of invertebrate muscle thick filaments. To investigate the importance of paramyosin phosphorylation, we produced transgenic Drosophila melanogaster in which one, three, or four phosphorylatable serine residues in the N-terminal nonhelical domain were replaced by alanines. Depending on the residues mutated, transgenic lines were either unaffected or severely flight impaired. Flight-impaired strains had decreases in the most acidic paramyosin isoforms, with a corresponding increase in more basic isoforms. Surprisingly, ultrastructure of indirect flight muscle myofibrils was normal, indicating N-terminal phosphorylation is not important for myofibril assembly. However, mechanical studies of active indirect flight muscle fibers revealed that phosphorylation site mutations reduced elastic and viscous moduli by 21-59% and maximum power output by up to 42%. Significant reductions also occurred under relaxed and rigor conditions, indicating that the phosphorylation-dependent changes are independent of strong crossbridge attachment and likely arise from alterations in thick filament backbone properties. Further, normal crossbridge kinetics were observed, demonstrating that myosin motor function is unaffected in the mutants. We conclude that N-terminal phosphorylation of Drosophila paramyosin is essential for optimal force and oscillatory power transduction within the muscle fiber and is key to the high passive stiffness of asynchronous insect flight muscles. Phosphorylation may reinforce interactions between myosin rod domains, enhance thick filament connections to the central M-line of the sarcomere and/or stabilize thick filament interactions with proteins that contribute to fiber stiffness.
Figures


Similar articles
-
Passive stiffness in Drosophila indirect flight muscle reduced by disrupting paramyosin phosphorylation, but not by embryonic myosin S2 hinge substitution.Biophys J. 2006 Dec 15;91(12):4500-6. doi: 10.1529/biophysj.106.088492. Epub 2006 Sep 29. Biophys J. 2006. PMID: 17012313 Free PMC article.
-
Overexpression of miniparamyosin causes muscle dysfunction and age-dependant myofibril degeneration in the indirect flight muscles of Drosophila melanogaster.J Muscle Res Cell Motil. 2001;22(3):287-99. doi: 10.1023/a:1012431725009. J Muscle Res Cell Motil. 2001. PMID: 11763201
-
Nature's strategy for optimizing power generation in insect flight muscle.Adv Exp Med Biol. 2005;565:157-66; discussion 167, 371-7. doi: 10.1007/0-387-24990-7_12. Adv Exp Med Biol. 2005. PMID: 16106973
-
The function of elastic proteins in the oscillatory contraction of insect flight muscle.J Muscle Res Cell Motil. 2005;26(6-8):479-85. doi: 10.1007/s10974-005-9032-7. J Muscle Res Cell Motil. 2005. PMID: 16450058 Review.
-
Determining structure/function relationships for sarcomeric myosin heavy chain by genetic and transgenic manipulation of Drosophila.Microsc Res Tech. 2000 Sep 15;50(6):430-42. doi: 10.1002/1097-0029(20000915)50:6<430::AID-JEMT2>3.0.CO;2-E. Microsc Res Tech. 2000. PMID: 10998634 Review.
Cited by
-
Aging and Autophagic Function Influences the Progressive Decline of Adult Drosophila Behaviors.PLoS One. 2015 Jul 16;10(7):e0132768. doi: 10.1371/journal.pone.0132768. eCollection 2015. PLoS One. 2015. PMID: 26182057 Free PMC article.
-
Alternative S2 hinge regions of the myosin rod affect myofibrillar structure and myosin kinetics.Biophys J. 2009 May 20;96(10):4132-43. doi: 10.1016/j.bpj.2009.02.034. Biophys J. 2009. PMID: 19450484 Free PMC article.
-
Immune system modulation & virus transmission during parasitism identified by multi-species transcriptomics of a declining insect biocontrol system.BMC Genomics. 2024 Mar 26;25(1):311. doi: 10.1186/s12864-024-10215-3. BMC Genomics. 2024. PMID: 38532315 Free PMC article.
-
Calcium signalling indicates bilateral power balancing in the Drosophila flight muscle during manoeuvring flight.J R Soc Interface. 2013 Mar 13;10(82):20121050. doi: 10.1098/rsif.2012.1050. Print 2013 May 6. J R Soc Interface. 2013. PMID: 23486171 Free PMC article.
-
Invertebrate muscles: thin and thick filament structure; molecular basis of contraction and its regulation, catch and asynchronous muscle.Prog Neurobiol. 2008 Oct;86(2):72-127. doi: 10.1016/j.pneurobio.2008.06.004. Epub 2008 Jun 20. Prog Neurobiol. 2008. PMID: 18616971 Free PMC article. Review.
References
-
- Winkelman, L. (1976) Comp. Biochem. Physiol. B 55, 391-397. - PubMed
-
- Beinbrech, G., Meller, U. & Wolfgang, S. (1985) Cell Tissue Res. 241, 607-614.
-
- Bullard, B., Luke, B. & Winkelman, L. (1973) J. Mol. Biol. 75, 359-367. - PubMed
-
- Vinos, J., Domingo, A., Marco, R. & Cervera, M. (1991) J. Mol. Biol. 220, 687-700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

