Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks
- PMID: 16020754
- PMCID: PMC1352308
- DOI: 10.1161/01.RES.0000177669.29525.78
Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks
Abstract
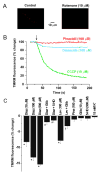
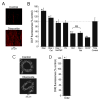
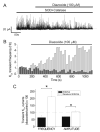
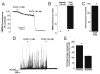
Mitochondria regulate intracellular calcium (Ca2+) signals in smooth muscle cells, but mechanisms mediating these effects, and the functional relevance, are poorly understood. Similarly, antihypertensive ATP-sensitive potassium (KATP) channel openers (KCOs) activate plasma membrane KATP channels and depolarize mitochondria in several cell types, but the contribution of each of these mechanisms to vasodilation is unclear. Here, we show that cerebral artery smooth muscle cell mitochondria are most effectively depolarized by diazoxide (-15%, tetramethylrhodamine [TMRM]), less so by levcromakalim, and not depolarized by pinacidil. KCO-induced mitochondrial depolarization increased the generation of mitochondria-derived reactive oxygen species (ROS) that stimulated Ca2+ sparks and large-conductance Ca2+-activated potassium (KCa) channels, leading to transient KCa current activation. KCO-induced mitochondrial depolarization and transient KCa current activation were attenuated by 5-HD and glibenclamide, KATP channel blockers. MnTMPyP, an antioxidant, and Ca2+ spark and KCa channel blockers reduced diazoxide-induced vasodilations by >60%, but did not alter dilations induced by pinacidil, which did not elevate ROS. Data suggest diazoxide drives ROS generation by inducing a small mitochondrial depolarization, because nanomolar CCCP, a protonophore, similarly depolarized mitochondria, elevated ROS, and activated transient KCa currents. In contrast, micromolar CCCP, or rotenone, an electron transport chain blocker, induced a large mitochondrial depolarization (-84%, TMRM), reduced ROS, and inhibited transient KCa currents. In summary, data demonstrate that mitochondria-derived ROS dilate cerebral arteries by activating Ca2+ sparks, that some antihypertensive KCOs dilate by stimulating this pathway, and that small and large mitochondrial depolarizations lead to differential regulation of ROS and Ca2+ sparks.
Figures



Comment in
-
Mitochondria and reactive oxygen species: an evolution in function.Circ Res. 2005 Aug 19;97(4):302-4. doi: 10.1161/01.RES.0000179773.18195.12. Circ Res. 2005. PMID: 16109924 No abstract available.
References
-
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol. 2000;278:C235–C256. - PubMed
-
- Perez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol. 2001;281:C1769–C1775. - PubMed
-
- Jaggar JH, Leffler CW, Cheranov SY, Tcheranova DES, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. - PubMed
-
- Wang YX, Zheng YM, Abdullaev I, Kotlikoff MI. Metabolic inhibition with cyanide induces calcium release in pulmonary artery myocytes and Xenopus oocytes. Am J Physiol. 2003;284:C378–C388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

