Guanine-specific oxidation of double-stranded DNA by Cr(VI) and ascorbic acid forms spiroiminodihydantoin and 8-oxo-2'-deoxyguanosine
- PMID: 16022506
- PMCID: PMC1305915
- DOI: 10.1021/tx050033y
Guanine-specific oxidation of double-stranded DNA by Cr(VI) and ascorbic acid forms spiroiminodihydantoin and 8-oxo-2'-deoxyguanosine
Abstract
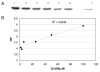
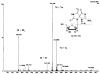
7,8-dihydro-8-oxoguanine (8-oxoG) is thought to be a major lesion formed in DNA by oxidative attack at the nucleobase guanine. Recent studies have shown that 8-oxoG has a lower reduction potential than the parent guanine and is a hot spot for further oxidation. Spiroiminodihydantoin (Sp) has been identified as one of these further oxidation products. Chromium(VI) is a human carcinogen that, when reduced by a cellular reductant such as ascorbate, can oxidize DNA. In this study, duplex DNA was reacted with Cr(VI) and ascorbate to identify and quantify the base lesions formed. Guanine bases were observed to be preferentially oxidized with 5' guanines within purine repeats showing enhanced oxidation. Trapping of the guanine lesions by the base excision repair enzymes hOGG1 and mNEIL2 showed nearly exclusive trapping by mNEIL2, suggesting that 8-oxoG was not the major lesion but rather a lesion recognized by mNEIL2 such as Sp. Formation of the Sp lesion in the Cr(VI)/Asc oxidation reaction with DNA was confirmed by LC-ESI-MS detection. HPLC-ECD was used to identify and quantify any 8-oxoG arising from Cr(VI)/Asc oxidation of DNA. Concentrations of Cr(VI) (3.1-50 microM) with a corresponding 1:10 ratio of Asc oxidized between 0.3% and 1.5% of all guanines within the duplex DNA strand to Sp. 8-oxoG was also identified but with the highest Cr(VI) concentration converting approximately 0.1% of all guanines to 8-oxoG. These results show that Sp was present in concentrations approximately 20 times greater than that of 8-oxoG in this system. The results indicate that 8-oxoG, while present, was not the major product of Cr(VI)/Asc oxidation of DNA and that Sp predominates under these conditions. These results further imply that Sp may be the lesion that accounts for the carcinogenicity of this metal in cellular systems.
Figures









References
-
- Hayes, R. B. (1982) Carcinogenic effects of chromium. In Biological and Environmental Aspects of Chromium (Langard, S., Ed.) pp 221–248, Elsevier Biomedical Press, New York.
-
- Miller CA, Costa M. Characterization of DNA-protein complexes induced in intact cells by the carcinogen chromate. Mol Carcinog. 1988;1:125–133. - PubMed
-
- Cantoni O, Costa M. Analysis of the induction of alkali sensitive sites in the DNA by chromate and other agents that induce single strand breaks. Carcinogenesis. 1984;5:1207–1209. - PubMed
-
- Cupo DY, Wetterhahn KE. Binding of chromium to chromatin and DNA from liver and kidney of rats treated with sodium dichromate and chromium(III) chloride in vivo. Cancer Res. 1985;145:1146–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

