SARS vaccine development
- PMID: 16022774
- PMCID: PMC3371787
- DOI: 10.3201/1107.050219
SARS vaccine development
Abstract
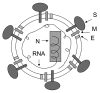
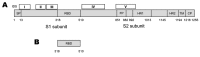
Developing effective and safe vaccines is urgently needed to prevent infection by severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV). The inactivated SARS-CoV vaccine may be the first one available for clinical use because it is easy to generate; however, safety is the main concern. The spike (S) protein of SARS-CoV is the major inducer of neutralizing antibodies, and the receptor-binding domain (RBD) in the S1 subunit of S protein contains multiple conformational neutralizing epitopes. This suggests that recombinant proteins containing RBD and vectors encoding the RBD sequence can be used to develop safe and effective SARS vaccines.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
