AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters
- PMID: 16022872
- PMCID: PMC3816113
- DOI: 10.1016/j.expneurol.2005.03.007
AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters
Abstract
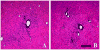
In this study, a modified infusion procedure and a novel infusion device designed for use in humans (Clinical Device B) were evaluated for delivery of recombinant adeno-associated virus (AAV2) to brain. The device is composed of 1.2 m of fused silica inserted through a 24.6-cm surgical steel cannula designed to fit a standard Leksell clinical stereotaxic frame and micro-infusion syringe pump. AAV2 encoding the human aromatic l-amino acid decarboxylase gene (AAV-hAADC-2) was infused into the putamen of 4 normal rhesus monkeys as a supportive study for a clinical trial in Parkinson's disease (PD) patients. Two infusion protocols were tested: a ramped procedure (slow stepwise increases in rate from 0.2 muL/min to 1 muL/min), thought to be essential for convection-enhanced delivery (CED), and a non-ramped infusion at a constant rate of 1 muL/min. The primary endpoints were safety evaluation of the infusion procedures and assessment of transgene expression at 5.5 weeks post-infusion. Clinical observations after vector infusions revealed no behavioral abnormalities during the study period. No differences in gross pathology with either the ramped or non-ramped infusion procedure were observed. Histopathology of the putamen was comparable with both procedures, and revealed only minimal localized inflammatory tissue reaction along the needle track in response to cannula placement and vector infusion. AADC immunohistochemistry demonstrated that vector was distributed throughout the putamen, with no significant difference in volume of immunostaining with either infusion procedure. Serum antibody levels against AAV2 vector exhibited a minor increase after infusion. These results validate the clinical utility of this new infusion device and non-ramped infusion conditions for intraputamenal gene therapy, and have the potential to impact a number of human diseases in which delivery of therapeutics to brain is indicated.
Figures
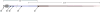
 ) is composed of 4 layers of 304 surgical steel fused together by laser welding in a step design, ending in 23-gauge tubing. The steel cannula is lined with fused silica of 100 Am inner diameter (
) is composed of 4 layers of 304 surgical steel fused together by laser welding in a step design, ending in 23-gauge tubing. The steel cannula is lined with fused silica of 100 Am inner diameter (
 , red) which also forms the tip of the delivery device by extending 1 cm beyond the steel. Approximately 1.2 m of additional fused silica covered with Teflon tubing (
, red) which also forms the tip of the delivery device by extending 1 cm beyond the steel. Approximately 1.2 m of additional fused silica covered with Teflon tubing (
 , blue) connect to a Luer hub (
, blue) connect to a Luer hub (
 ). A 1-in., 23-gauge steel spacer (
). A 1-in., 23-gauge steel spacer (
 ) between the fused silica and Teflon tubing is sealed and attached to the Luer hub with medical grade cyanoacrylate glue. Dimensions are in centimeters.
) between the fused silica and Teflon tubing is sealed and attached to the Luer hub with medical grade cyanoacrylate glue. Dimensions are in centimeters.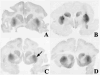

Similar articles
-
Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain.Hum Gene Ther. 2006 Mar;17(3):291-302. doi: 10.1089/hum.2006.17.291. Hum Gene Ther. 2006. PMID: 16544978
-
Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain.Mol Ther. 2008 Jul;16(7):1267-75. doi: 10.1038/mt.2008.111. Epub 2008 Jun 3. Mol Ther. 2008. PMID: 18523450 Free PMC article.
-
Carbidopa-based modulation of the functional effect of the AAV2-hAADC gene therapy in 6-OHDA lesioned rats.PLoS One. 2015 Apr 10;10(4):e0122708. doi: 10.1371/journal.pone.0122708. eCollection 2015. PLoS One. 2015. PMID: 25860990 Free PMC article.
-
Intraparenchymal delivery of adeno-associated virus vectors for the gene therapy of neurological diseases.Expert Opin Biol Ther. 2024 Aug;24(8):773-785. doi: 10.1080/14712598.2024.2386339. Epub 2024 Jul 31. Expert Opin Biol Ther. 2024. PMID: 39066718 Review.
-
Gene therapy of Parkinson's disease using adeno-associated virus (AAV) vectors.J Neural Transm Suppl. 2000;(58):181-91. doi: 10.1007/978-3-7091-6284-2_15. J Neural Transm Suppl. 2000. PMID: 11128607 Review.
Cited by
-
Adeno-Associated Virus-Based Gene Therapy for CNS Diseases.Hum Gene Ther. 2016 Jul;27(7):478-96. doi: 10.1089/hum.2016.087. Hum Gene Ther. 2016. PMID: 27267688 Free PMC article. Review.
-
Safety and tolerability of putaminal AADC gene therapy for Parkinson disease.Neurology. 2009 Nov 17;73(20):1662-9. doi: 10.1212/WNL.0b013e3181c29356. Epub 2009 Oct 14. Neurology. 2009. PMID: 19828868 Free PMC article. Clinical Trial.
-
Injection parameters and virus dependent choice of promoters to improve neuron targeting in the nonhuman primate brain.Gene Ther. 2014 Mar;21(3):233-41. doi: 10.1038/gt.2013.75. Epub 2014 Jan 9. Gene Ther. 2014. PMID: 24401836 Free PMC article.
-
Six-month partial suppression of Huntingtin is well tolerated in the adult rhesus striatum.Brain. 2012 Apr;135(Pt 4):1197-209. doi: 10.1093/brain/awr333. Epub 2012 Jan 16. Brain. 2012. PMID: 22252996 Free PMC article.
-
Genome concentration, characterization, and integrity analysis of recombinant adeno-associated viral vectors using droplet digital PCR.PLoS One. 2023 Jan 25;18(1):e0280242. doi: 10.1371/journal.pone.0280242. eCollection 2023. PLoS One. 2023. PMID: 36696399 Free PMC article.
References
-
- Bankiewicz KS, Eberling JL, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164(1):2–14. - PubMed
-
- Blacklow NR, Hoggan MD, et al. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40(2):319–327. - PubMed
-
- Grimm D, Kern A, et al. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9(18):2745–2760. - PubMed
-
- Hornykiewicz O. Brain monoamines and parkinsonism. Natl Inst Drug Abuse Res Monogr Ser. 1975;3:13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

