Multidimensional separations of ubiquitin conformers in the gas phase: relating ion cross sections to H/D exchange measurements
- PMID: 16023362
- PMCID: PMC2735248
- DOI: 10.1016/j.jasms.2005.04.007
Multidimensional separations of ubiquitin conformers in the gas phase: relating ion cross sections to H/D exchange measurements
Abstract
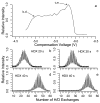
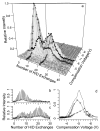
Investigating gas-phase structures of protein ions can lead to an improved understanding of intramolecular forces that play an important role in protein folding. Both hydrogen/deuterium (H/D) exchange and ion mobility spectrometry provide insight into the structures and stabilities of different gas-phase conformers, but how best to relate the results from these two methods has been hotly debated. Here, high-field asymmetric waveform ion mobility spectrometry (FAIMS) is combined with Fourier-transform ion cyclotron resonance mass spectrometry (FT/ICR MS) and is used to directly relate ubiquitin ion cross sections and H/D exchange extents. Multiple conformers can be identified using both methods. For the 9+ charge state of ubiquitin, two conformers (or unresolved populations of conformers) that have cross sections differing by 10% are resolved by FAIMS, but only one conformer is apparent using H/D exchange at short times. For the 12+ charge state, two conformers (or conformer populations) have cross sections differing by <1%, yet H/D exchange of these conformers differ significantly (6 versus 25 exchanges). These and other results show that ubiquitin ion collisional cross sections and H/D exchange distributions are not strongly correlated and that factors other than surface accessibility appear to play a significant role in determining rates and extents of H/D exchange. Conformers that are not resolved by one method could be resolved by the other, indicating that these two methods are highly complementary and that more conformations can be resolved with this combination of methods than by either method alone.
Figures





References
-
- Onuchic JN, LutheySchulten Z, Wolynes PG. Theory of Protein Folding: The Energy Landscape Perspective. Annu Rev Phys Chem. 1997;48:545–600. - PubMed
- Onuchic JN, Wolynes PG. Theory of Protein Folding. Curr Opin Struct Biol. 2004;14:70–75. - PubMed
- Kubelka J, Hofrichter J, Eaton EA. The Protein Folding “Speel Limit”. Curr Opin Struct Biol. 2004;14:76–88. - PubMed
-
- Jarrold MF. Peptides and Proteins in the Vapor Phase. Ann Rev Phys Chem. 2000;51:179–207. - PubMed
-
- Suckau D, Shi Y, Beu SC, Senko MW, Quinn JP, Wampler FM, McLafferty FW. Coexisting Stable Conformations of Gaseous Protein Ions. Proc Natl Acad Sci USA. 1993;90:790–793. - PMC - PubMed
- McLafferty FW, Guan Z, Haupts U, Wood TD, Kelleher NL. Gaseous Conformational Structures of Cytochrome. c J Am Chem Soc. 1998;120:4732–4740.
- Wood TD, Chorush RA, Wampler FM, Little DP, O’Connor PB, McLafferty FW. Gas-Phase Folding and Unfolding of Cytochrome c Cations. Proc Natl Acad Sci USA. 1995;92:2451–2454. - PMC - PubMed
-
- Freitas MA, Hendrickson CL, Emmett MR, Marshall AG. Gas-Phase Bovine Ubiquitin Cation Conformations Resolved by Gas-Phase Hydrogen/Deuterium Exchange Rate and Extent. Int J Mass Spectrom. 1999;185/186/187:565–575.
-
- Winger BE, Light-Wahl KJ, Rockwood AL, Smith RD. Probing Qualitative Conformation Differences of Multiply Protonated Gas-Phase Proteins via H/D Isotopic Exchange with D2O. J Am Chem Soc. 1992;114:5897–5898.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

