Purified mouse dopamine neurons thrive and function after transplantation into brain but require novel glial factors for survival in culture
- PMID: 16024255
- PMCID: PMC1949425
- DOI: 10.1016/j.mcn.2005.06.004
Purified mouse dopamine neurons thrive and function after transplantation into brain but require novel glial factors for survival in culture
Erratum in
- Mol Cell Neurosci. 2005 Dec;30(4):559
Corrected and republished in
-
Purified mouse dopamine neurons thrive and function after transplantation into brain but require novel glial factors for survival in culture.Mol Cell Neurosci. 2005 Dec;30(4):601-10. Mol Cell Neurosci. 2005. PMID: 16456927
Abstract
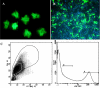
Cell replacement therapy in Parkinson's disease depends on a reliable source of purified dopamine (DA) neurons (PDN) and the identification of factors relevant to their survival. Our goal was to genetically tag and purify by flow cytometry embryonic midbrain DA neurons from a transgenic mouse line carrying 11 kb of human tyrosine hydroxylase promoter driving expression of the enhanced green fluorescent protein (GFP) for studies in vivo and in vitro. A 99% purification of GFP(+) cells was achieved. When transplanted into 6-hydroxydopamine-treated rat striatum, PDN survived, became well-integrated and produced recovery from amphetamine-induced motor behaviors. However, when grown in culture, PDN died within days of plating. No known growth factors prevented PDN death as did incubation with novel factors in glia/glial-conditioned media. We conclude that GFP-tagged DA neurons can be purified to homogeneity and can survive and function when grown with glial factors in vitro or after transplantation in vivo.
Figures






References
-
- Akerud P, Alberch J, Eketjall S, Wagner J, Arenas E. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J. Neurochem. 1999;73:70–78. - PubMed
-
- Barker RA, Dunnett SB, Faissner A, Fawcett JW. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp. Neurol. 1996;141:79–93. - PubMed
-
- Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, Horellou P. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8818–8823. - PMC - PubMed
-
- Bjorklund LM, Sanchez-Pernaute R, Chung S, Anderson T, Chen IYC, McNaught KSP, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinsonian rat model. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2344–2349. - PMC - PubMed
-
- Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RF. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell. Transplant. 2000;9:179–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

