Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha
- PMID: 16024780
- PMCID: PMC1190339
- DOI: 10.1128/MCB.25.15.6415-6426.2005
Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha
Abstract
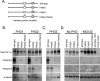
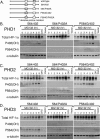
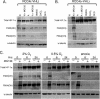
Oxygen-dependent proteolysis is the primary means of regulating the hypoxia-inducible factor (HIF) family of transcription factors. The alpha-subunit of HIF factor 1 (HIF-1) contains two highly conserved oxygen-dependent degradation domains (402 ODD and 564 ODD), each of which includes a proline that is hydroxylated in the presence of oxygen, allowing the von Hippel-Lindau (VHL) E3 ubiquitin ligase to interact and target HIF-1alpha to the proteasome for degradation. Mutation of either proline is sufficient to partially stabilize HIF-1alpha under conditions of normoxia, but the specific contributions of each hydroxylation event to the regulation of HIF-1alpha are unknown. Here we show that the two ODDs of HIF-1alpha have independent yet interactive roles in the regulation of HIF-1alpha protein turnover, with the relative involvement of each ODD depending on the levels of oxygen. Using hydroxylation-specific antibodies, we found that under conditions of normoxia proline 564 is hydroxylated prior to proline 402, and mutation of proline 564 results in a significant reduction in the hydroxylation of proline 402. Mutation of proline 402, however, has little effect on the hydroxylation of proline 564. To determine whether the more rapid hydroxylation of the proline 564 under conditions of normoxia is due to a preference for the particular sequence surrounding proline 564 or for that site within the protein, we exchanged the degradation domains within the full-length HIF-1alpha protein. In these domain-swapping experiments, prolyl hydroxylase domain 1 (PHD1) and PHD2 preferentially hydroxylated the proline located in the site of the original 564 ODD, while PHD3 preferred the proline 564 sequence, regardless of its location. At limiting oxygen tensions, we found that proline 402 exhibits an oxygen-dependent decrease in hydroxylation at higher oxygen tensions relative to proline 564 hydroxylation. These results indicate that hydroxylation of proline 402 is highly responsive to physiologic changes in oxygen and, therefore, plays a more important role in HIF-1alpha regulation under conditions of hypoxia than under conditions of normoxia. Together, these findings demonstrate that each hydroxylated proline of HIF-1alpha has a distinct activity in controlling HIF-1alpha stability in response to different levels of oxygenation.
Figures




Similar articles
-
A common polymorphism in the oxygen-dependent degradation (ODD) domain of hypoxia inducible factor-1alpha (HIF-1alpha) does not impair Pro-564 hydroxylation.Mol Cancer. 2003 Sep 9;2:31. doi: 10.1186/1476-4598-2-31. Mol Cancer. 2003. PMID: 14521712 Free PMC article.
-
Leu-574 of human HIF-1alpha is a molecular determinant of prolyl hydroxylation.FASEB J. 2004 Jun;18(9):1028-30. doi: 10.1096/fj.03-1233fje. Epub 2004 Apr 14. FASEB J. 2004. PMID: 15084514
-
Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases.Biochem J. 2004 Aug 1;381(Pt 3):761-7. doi: 10.1042/BJ20040620. Biochem J. 2004. PMID: 15104534 Free PMC article.
-
Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions.Exp Mol Med. 2004 Feb 29;36(1):1-12. doi: 10.1038/emm.2004.1. Exp Mol Med. 2004. PMID: 15031665 Review.
-
Hypoxia-inducible factor 1 (HIF-1) pathway.Sci STKE. 2007 Oct 9;2007(407):cm8. doi: 10.1126/stke.4072007cm8. Sci STKE. 2007. PMID: 17925579 Review.
Cited by
-
ATF4 and HIF-1α in bone: an intriguing relationship.J Bone Miner Res. 2013 Sep;28(9):1866-9. doi: 10.1002/jbmr.2045. J Bone Miner Res. 2013. PMID: 23873659 Free PMC article. No abstract available.
-
Dysregulated glycolysis as an oncogenic event.Cell Mol Life Sci. 2015 May;72(10):1881-92. doi: 10.1007/s00018-015-1840-3. Epub 2015 Jan 22. Cell Mol Life Sci. 2015. PMID: 25609364 Free PMC article. Review.
-
Identification of a region on hypoxia-inducible-factor prolyl 4-hydroxylases that determines their specificity for the oxygen degradation domains.Biochem J. 2007 Dec 1;408(2):231-40. doi: 10.1042/BJ20071052. Biochem J. 2007. PMID: 17725546 Free PMC article.
-
The Role of VHL in the Development of von Hippel-Lindau Disease and Erythrocytosis.Genes (Basel). 2022 Feb 17;13(2):362. doi: 10.3390/genes13020362. Genes (Basel). 2022. PMID: 35205407 Free PMC article. Review.
-
Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed In Vivo and In Vitro.Int J Environ Res Public Health. 2021 Oct 29;18(21):11374. doi: 10.3390/ijerph182111374. Int J Environ Res Public Health. 2021. PMID: 34769890 Free PMC article. Review.
References
-
- Appelhoff, R. J., Y. M. Tian, R. R. Raval, H. Turley, A. L. Harris, C. W. Pugh, P. J. Ratcliffe, and J. M. Gleadle. 2004. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279:38458-38465. - PubMed
-
- Baek, J. H., P. C. Mahon, J. Oh, B. Kelly, B. Krishnamachary, M. Pearson, D. A. Chan, A. J. Giaccia, and G. L. Semenza. 2005. OS-9 interacts with hypoxia-inducible factor 1alpha and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1alpha. Mol. Cell 17:503-512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
