Transformation of human and murine fibroblasts without viral oncoproteins
- PMID: 16024784
- PMCID: PMC1190359
- DOI: 10.1128/MCB.25.15.6464-6474.2005
Transformation of human and murine fibroblasts without viral oncoproteins
Abstract
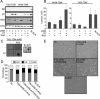
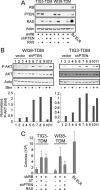
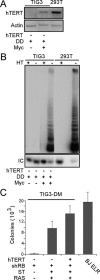
Murine embryo fibroblasts are readily transformed by the introduction of specific combinations of oncogenes; however, the expression of those same oncogenes in human cells fails to convert such cells to tumorigenicity. Using normal human and murine embryonic fibroblasts, we show that the transformation of human cells requires several additional alterations beyond those required to transform comparable murine cells. The introduction of the c-Myc and H-RAS oncogenes in the setting of loss of p53 function efficiently transforms murine embryo fibroblasts but fails to transform human cells constitutively expressing hTERT, the catalytic subunit of telomerase. In contrast, transformation of multiple strains of human fibroblasts requires the constitutive expression of c-Myc, H-RAS, and hTERT, together with loss of function of the p53, RB, and PTEN tumor suppressor genes. These manipulations permit the development of transformed human fibroblasts with genetic alterations similar to those found associated with human cancers and define specific differences in the susceptibility of human and murine fibroblasts to experimental transformation.
Figures



References
-
- Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA 93:5699-5704. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
