The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages
- PMID: 16025156
- PMCID: PMC1174916
- DOI: 10.1172/JCI23755
The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages
Abstract
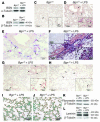
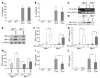
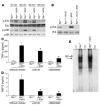
Biglycan, a small leucine-rich proteoglycan, is a ubiquitous ECM component; however, its biological role has not been elucidated in detail. Here we show that biglycan acts in macrophages as an endogenous ligand of TLR4 and TLR2, which mediate innate immunity, leading to rapid activation of p38, ERK, and NF-kappaB and thereby stimulating the expression of TNF-alpha and macrophage inflammatory protein-2 (MIP-2). In agreement, the stimulatory effects of biglycan are significantly reduced in TLR4-mutant (TLR4-M), TLR2-/-, and myeloid differentiation factor 88-/- (MyD88-/-) macrophages and completely abolished in TLR2-/-/TLR4-M macrophages. Biglycan-null mice have a considerable survival benefit in LPS- or zymosan-induced sepsis due to lower levels of circulating TNF-alpha and reduced infiltration of mononuclear cells in the lung, which cause less end-organ damage. Importantly, when stimulated by LPS-induced proinflammatory factors, macrophages themselves are able to synthesize biglycan. Thus, biglycan, upon release from the ECM or from macrophages, can boost inflammation by signaling through TLR4 and TLR2, thereby enhancing the synthesis of TNF-alpha and MIP-2. Our results provide evidence for what is, to our knowledge, a novel role of the matrix component biglycan as a signaling molecule and a crucial proinflammatory factor. These findings are potentially relevant for the development of new strategies in the treatment of sepsis.
Figures


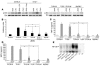
References
-
- Iozzo R. The biology of the small leucine-rich proteoglycans: functional network of interactive proteins [review] J. Biol. Chem. 1999;274:18843–18846. - PubMed
-
- Ameye L, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. - PubMed
-
- Xu T, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat. Genet. 1998;20:78–82. - PubMed
-
- Weber C, et al. Biglycan is overexpressed in pancreatic cancer and induces G1-arrest in pancreatic cancer cell lines. Gastroenterology. 2001;121:657–667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

