Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients
- PMID: 16025158
- PMCID: PMC1174914
- DOI: 10.1172/JCI23196
Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients
Abstract
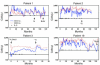
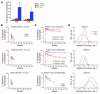
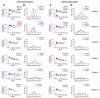
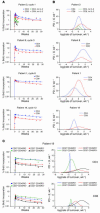
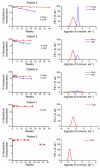
HIV infection leads to decreases in the number of CD4 T lymphocytes and an increased risk for opportunistic infections and neoplasms. The administration of intermittent cycles of IL-2 to HIV-infected patients can lead to profound increases (often greater than 100%) in CD4 cell number and percentage. Using in vivo labeling with 2H-glucose and BrdU, we have been able to demonstrate that, although therapy with IL-2 leads to high levels of proliferation of CD4 as well as CD8 lymphocytes, it is a remarkable preferential increase in survival of CD4 cells (with half-lives that can exceed 3 years) that is critical to the sustained expansion of these cells. This increased survival was time-dependent: the median half-life, as determined by semiempirical modeling, of labeled CD4 cells in 6 patients increased from 1.7 weeks following an early IL-2 cycle to 28.7 weeks following a later cycle, while CD8 cells showed no change in the median half-life. Examination of lymphocyte subsets demonstrated that phenotypically naive (CD27+CD45RO-) as well as central memory (CD27+CD45RO+) CD4 cells were preferentially expanded, suggesting that IL-2 can help maintain cells important for host defense against new antigens as well as for long-term memory to opportunistic pathogens.
Figures
References
-
- Kovacs JA, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 1996;335:1350–1356. - PubMed
-
- Davey RT, Jr, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: a randomized controlled trial. JAMA. 2000;284:183–189. - PubMed
-
- Levy Y, et al. Comparison of subcutaneous and intravenous interleukin-2 in asymptomatic HIV-1 infection: a randomised controlled trial. ANRS 048 study group. Lancet. 1999;353:1923–1929. - PubMed
-
- Kovacs JA, et al. Interleukin-2 induced immune effects in human immunodeficiency virus-infected patients receiving intermittent interleukin-2 immunotherapy. Eur. J. Immunol. 2001;31:1351–1360. - PubMed
-
- Davey RT, Jr, et al. A randomized trial of high- versus low-dose subcutaneous interleukin-2 outpatient therapy for early human immunodeficiency virus type 1 infection. J. Infect. Dis. 1999;179:849–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

