Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers
- PMID: 16025322
- PMCID: PMC4079466
- DOI: 10.1007/s00213-005-0023-6
Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers
Abstract
Rationale: Although buprenorphine is effective in treating opioid dependence, optimal maintenance doses of buprenorphine or the buprenorphine/naloxone combination have not yet been established.
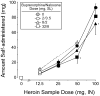
Objective: The present study was designed to evaluate the effects of buprenorphine/naloxone maintenance (2/0.5, 8/2, 32/8 mg sublingual) on the reinforcing and subjective effects of heroin (0, 12.5, 25, 50, and 100 mg intranasal) in heroin-dependent individuals.
Methods: During test weeks, participants (N=7) first sampled a dose of heroin and 20 dollars. During subsequent choice sessions, participants could choose to self-administer heroin and/or money. Participants responded under a modified progressive-ratio schedule (PR 50, ..., 2,800) during a ten-trial self-administration task.
Results: Heroin break point values and subjective responses were significantly lower under 8/2 and 32/8 mg buprenorphine/naloxone compared to 2/0.5 mg. The self-administration and subjective effects data for heroin in the presence of buprenorphine/naloxone were compared to a separate control group of recently detoxified participants (N=8) in order to obtain estimates for the apparent in vivo dissociation constant (K(A)), the efficacy estimate (tau), and the estimated fraction of receptors remaining after buprenorphine/naloxone treatment (q). The apparent in vivo dissociation constant for heroin ranged from 50 to 126 mg (K(A)) and the efficacy estimate ranged from 13 to 20 (tau). In addition, 2/0.5, 8/2, and 32/8 mg buprenorphine/naloxone dose-dependently reduced the receptor population by 74, 83, and 91%, respectively.
Conclusions: These data demonstrate that both 8/2 and 32/8 mg buprenorphine/naloxone were well tolerated and effective in reducing the reinforcing and subjective effects of heroin, relative to the 2/0.5-mg dose. The data also show for the first time in humans that it is possible to quantify the efficacy and affinity of heroin for mu opioid receptors, and that 80-90% of mu receptors need to be inactivated in order to obtain significant reductions in heroin-induced effects. These results have important implications for future studies in which it will be possible to obtain estimates of relative affinity and efficacy of different agonists at mu opioid receptors.
Figures

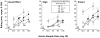
References
-
- Barrett AC, Smith ES, Picker MJ. Use of irreversible antagonists to determine the relative efficacy of mu opioids in a pigeon drug discrimination procedure: Comparison of beta-funaltrexamine and clocinnamox. J Pharmacol Exp Ther. 2003:1061–1070. - PubMed
-
- Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology. 1999;146:111–118. - PubMed
-
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial with buprenorphine: Comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther. 1988a;43:72–78. - PubMed
-
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: Dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988b;247:47–53. - PubMed
-
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B. 1983;220:141–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

