Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging
- PMID: 16026324
- PMCID: PMC2228271
- DOI: 10.2174/1570161054368616
Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging
Abstract
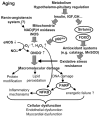
Epidemiological studies demonstrated that even in the absence of other risk factors (e.g. diabetes, hypertension, hypercholesterolemia), advanced age itself significantly increases cardiovascular morbidity. Although aging is inevitable, cardiovascular gerontologists recognize that a better understanding of the aging process in the not-so-distant future will lead to pharmacological interventions that considerably delay the functional decline of the cardiovascular system. Since the original publishing of the free radical theory of aging, an increased production of reactive oxygen species has been implicated both in the aging process and the development of age-related cardiovascular diseases. This review focuses on the role of oxidative and nitrosative stress in cardiovascular dysfunction in aging, downstream mechanisms including activation of NF- kappaB, and the role of poly(ADP-ribose)polymerase (PARP) and longevity genes that are linked to regulation of cellular redox status and oxidative stress resistance (p66(shc), sirtuins, FOXO transcription factors).
Figures


References
-
- Yang B, Larson DF, Watson RR. Modulation of iNOS activity in age-related cardiac dysfunction. Life Sci. 2004;75(6):655–67. - PubMed
-
- Harman D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J Gerontol. 1956;11:298–300. - PubMed
-
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159–66. - PubMed
-
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37(2):529–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

